Cefepime
General Information
Cefepime Impurities and Cefepime
Daicel Pharma synthesizes high-quality Cefepime impurity, Cefepime sulfoxides, which is crucial in analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient Cefepime. Moreover, Daicel Pharma offers custom synthesis of Cefepime impurities and delivers them globally.
Cefepime [CAS: 88040-23-7] is a fourth-generation cephalosporin that treats bacterial infections caused by gram-negative and gram-positive bacteria. It belongs to the beta-lactam class of antibiotics.
Cefepime: Use and Commercial Availability
Cefepime is a medicine recommended for treating pneumonia caused by susceptible bacteria. It acts as an initial therapy for patients with febrile neutropenia. Cefepime treats uncomplicated and complicated urinary tract infections (including pyelonephritis), uncomplicated skin and skin structure infections, and complicated intra-abdominal infections (when used with metronidazole).
Cefepime is available under the brand name such as Maxipime.
Cefepime Structure and Mechanism of Action 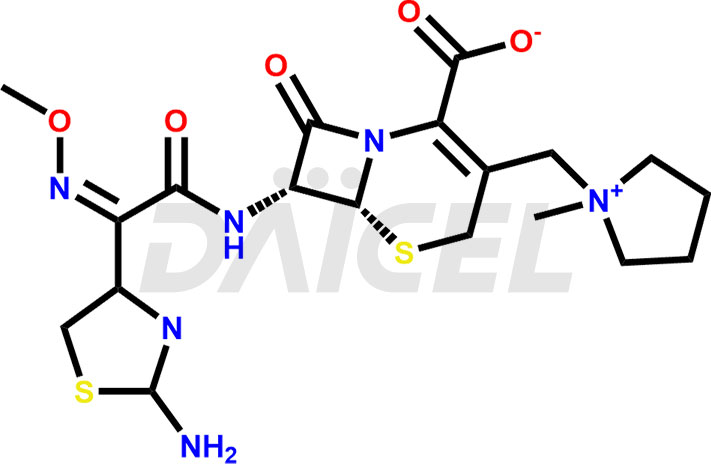
The chemical name of Cefepime is 1-[[(6R,7R)-7-[[(2Z)-2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methyl- Pyrrolidinium. Its chemical formula is C19H24N6O5S2, and its molecular weight is approximately 480.6 g/mol.
Cefepime is a bactericidal agent that inhibits bacterial cell wall synthesis. It is effective against Gram-positive and Gram-negative bacteria.
Cefepime Impurities and Synthesis
Cefepime impurities can be related substances, degradation products, or residual solvents formed during the drug’s manufacturing process1. It is crucial to monitor and control these impurities to ensure patient safety.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for the Cefepime impurity standard, Cefepime sulfoxides. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Cefepime impurity or degradation product.
References
- Aburaki, Shimpei; Kamachi, Hajime; Narita, Yukio; Okumura, Jun; Naito, Takayuki., Cephalosporins, Bristol-Myers Co., United States, US4406899A, September 27, 1983
- Barbhaiya, R. H.; Forgue, S. T.; Shyu, W. C.; Papp, E. A.; Pittman, K. A., High-pressure liquid-chromatographic analysis of BMY-28142 in plasma and urine, Antimicrobial Agents and Chemotherapy,Volume: 31, Issue: 1, Pages: 55-9, 1987
Frequently Asked Questions
Which analytical methods help identify and quantify Cefepime impurities?
Chromatographic techniques like LC-MS and HPLC help detect and analyze impurities in Cefepime.
What is the other commonly used technique to determine Cefepime impurities?
UV absorption spectroscopy is the other commonly used technique to determine impurities in Cefepime.
Which solvent is for the analysis of Cefepime impurities?
Methanol is a solvent to analyze Cefepime and its impurities.
What are the temperature conditions required to store Cefepime impurities?
Cefepime impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.


