LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma synthesizes more than ten high-quality Cefdinir impurities, including 3-methyl Cefdinir, Cefdinir Dimer, Cefdinir Glyoxalic Analog, Cefdinir Isoxazole Analog, Cefdinir Sulfoxide, E-Cefdinir, E-Cefdinir Lactone, Thiazolyl Acetyl Glycine Oxime, Thiazolyl acetyl glycine oxime acetal and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient, Cefdinir. Moreover, Daicel Pharma offers custom synthesis of Cefdinir impurities and delivers them globally.
Cefdinir [CAS: 91832-40-5] is a beta-lactam antibiotic. It is a semi-synthetic cephalosporin that exhibits bactericidal activity.
Cefdinir is commonly used to treat various bacterial infections in children and adults, including acute bacterial otitis media, community-acquired pneumonia, acute maxillary sinusitis, pharyngitis/tonsillitis, acute bacterial exacerbations of chronic bronchitis, uncomplicated skin, and skin structure infections. The drug can be effective against several organisms causing bacterial infections, such as Haemophilus influenzae, Haemophilus parainfluenzae, Streptococcus pneumoniae (penicillin-susceptible only), Moraxella catarrhalis, Streptococcus pyogenes, and Staphylococcus aureus. Cefdinir is available under the brand name Omnicef.
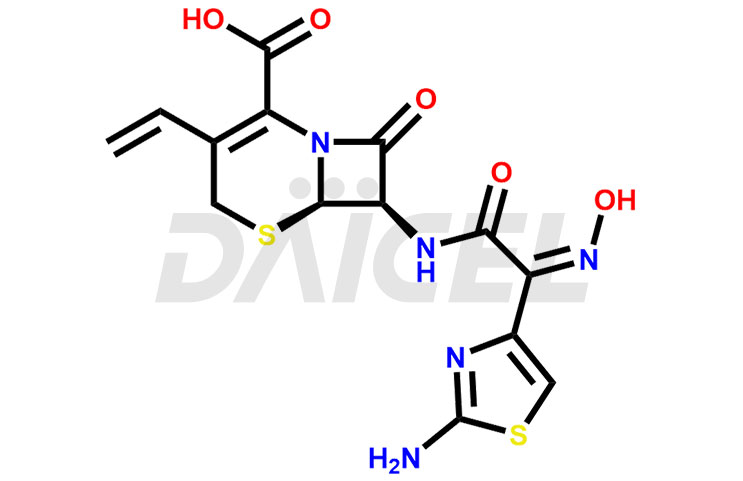
The chemical name of Cefdinir is (6R,7R)-7-[[(2Z)-2-(2-Amino-4-thiazolyl)-2-(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid . Its chemical formula is C14H13N5O5S2, and its molecular weight is approximately 395.4 g/mol.
Just like other cephalosporins, Cefdinir inhibits bacterial wall synthesis.
Various impurities form in Cefdinir, including related compounds, degradation products, solvent impurities, inorganic impurities, and residual reagents. These impurities occur during the manufacturing process1 or storage of the drug and can affect the safety and efficacy of the drug. It is vital to identify and quantify these impurities using analytical techniques and ensure that they are within acceptable regulatory limits to ensure the quality and stability of the drug.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for more than ten Cefdinir impurity standards, including 3-methyl Cefdinir, Cefdinir Dimer, Cefdinir Glyoxalic Analog, Cefdinir Isoxazole Analog, Cefdinir Sulfoxide, E-Cefdinir, E-Cefdinir Lactone, Thiazolyl Acetyl Glycine Oxime, Thiazolyl acetyl glycine oxime acetal and so on. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Cefdinir impurity or degradation product.
The effect of impurities on the shelf life of Cefdinir depends on the specific impurity class and the storage conditions of the drug. Impurities reduce the drug's potency and efficacy by degradation. Further, their presence in the drug product may also accelerate the breakdown of the drug, leading to a shorter shelf life.
Analytical techniques such as HPLC and LC-MS help monitor Cefdinir impurities during manufacturing.
DMSO is a solvent to analyze Cefdinir and its impurities.
Cefdinir impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.