Cefazolin
General Information
Cefazolin Impurities and Cefazolin
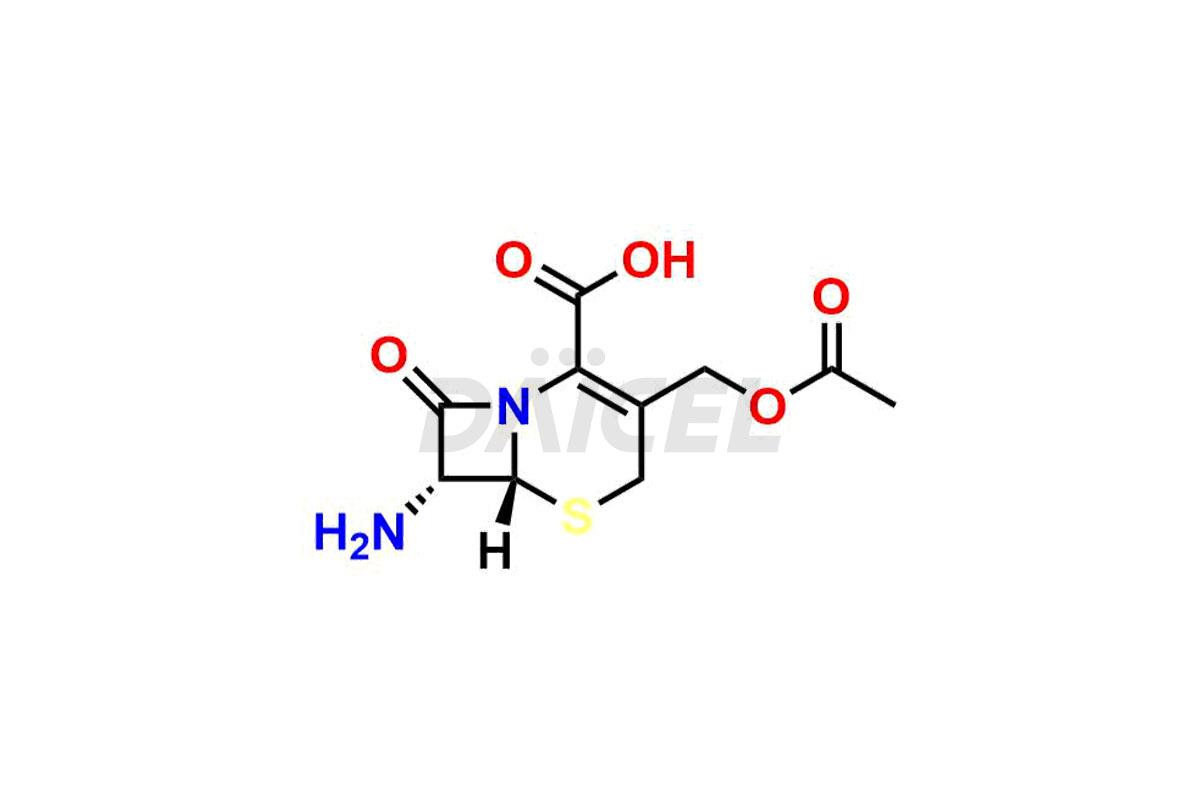
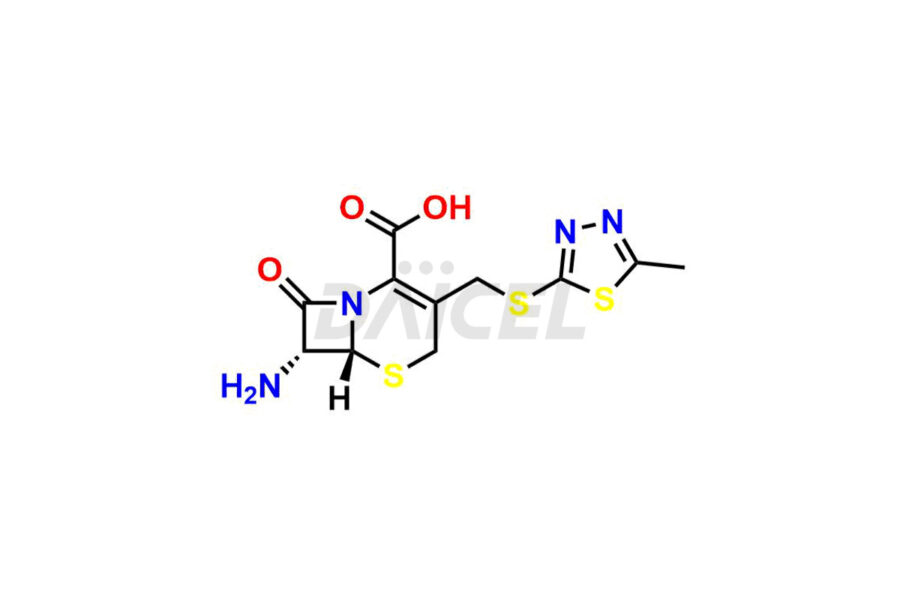
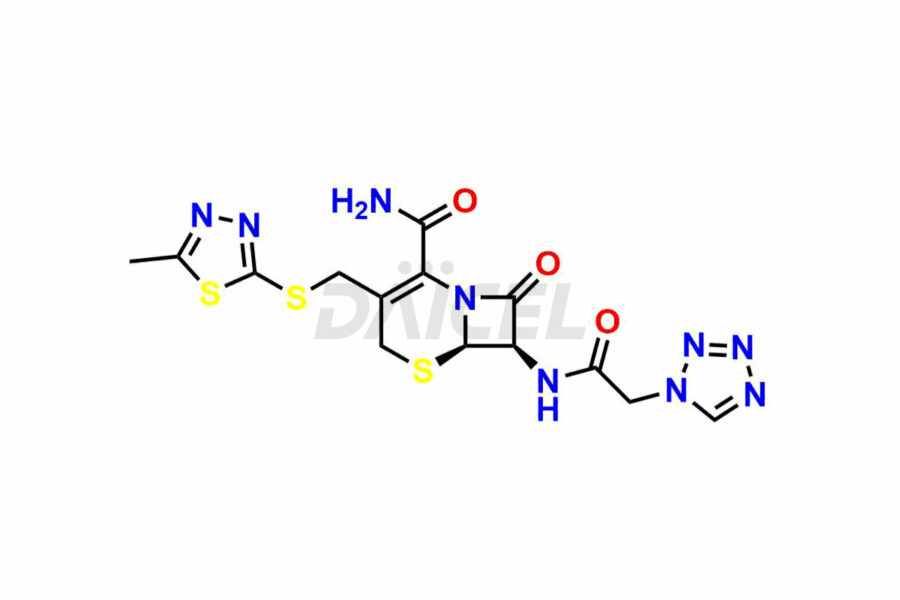
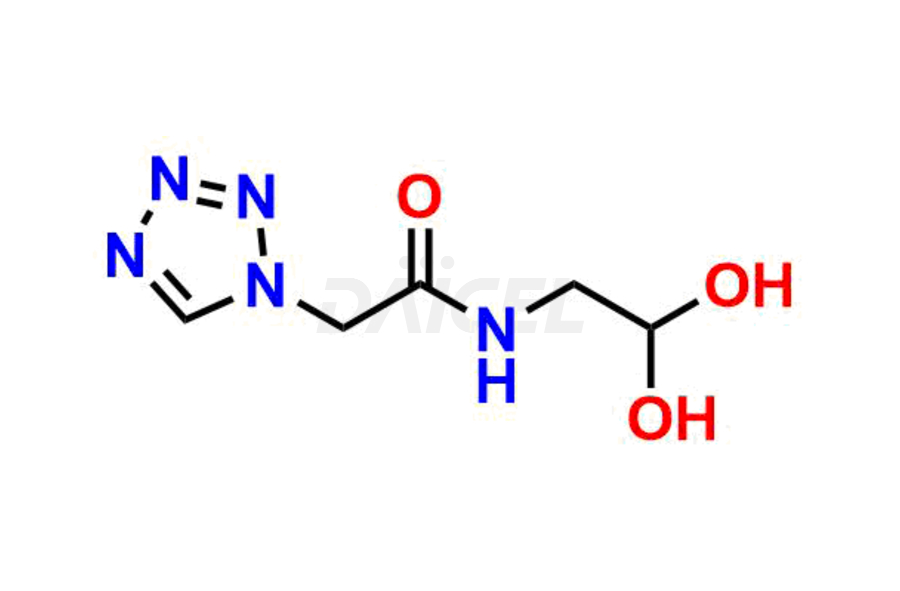
Daicel Pharma synthesizes high-quality Cefazolin impurities, 7-aminocephalosporanic acid, Cefazolin EP impurity A, Cefazolin EP Impurity-K and Tetrazolyl Acetamide Acetal, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient, Cefazolin. Moreover, Daicel Pharma offers custom synthesis of Cefazolin impurities and delivers them globally.
Cefazolin [CAS: 25953-19-9] is an antibiotic belonging to beta-lactam and first-generation cephalosporin. It has bactericidal activity.
Cefazolin: Use and Commercial Availability
Cefazolin is an antibiotic primarily for treating bacterial skin infections and moderately severe bacterial infections of the lung, bone, joint, stomach, blood, heart valve, and urinary tract. It is effective against Gram-positive bacteria, specifically staphylococci and streptococci species. The medicine is available under several trade names, including ANCEF, CEFAZOLIN SODIUM, and KEFZOL.
Cefazolin Structure and Mechanism of Action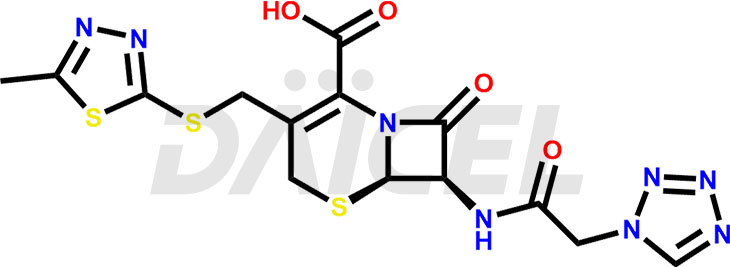
The chemical name of Cefazolin is (6R,7R)-3-[[(5-Methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[2-(1H-tetrazol-1-yl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Its chemical formula is C14H14N8O4S3, and its molecular weight is approximately 454.5 g/mol.
Cefazolin binds to penicillin-binding proteins (PBP) present on the inner membrane of bacterial cell walls, causing the weakening of the bacterial cell wall and cell lysis.
Cefazolin Impurities and Synthesis
The synthesis1,2 of Cefazolin can lead to the formation of impurities, including epimers, degradation products and more, that can affect the quality and safety of the drug. Manufacturers must employ strict quality control measures to minimize the formation of impurities and conduct regular testing to monitor impurity levels in Cefazolin.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Cefazolin impurity standards, 7-aminocephalosporanic acid, Cefazolin EP impurity A, Cefazolin EP Impurity-K and Tetrazolyl Acetamide Acetal. The CoA includes complete characterization data, such as 1H NMR, 13C NMR3, IR, MASS, and HPLC purity. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Cefazolin impurity or degradation product.
References
FAQ's
References
- Kariyone, Kazuo; Harada, Hirokichi; Kurita, Masaru; Takano, Tadayoshi, Cephalosporin. III. Cefazolin, a new semisynthetic cephalosporin antibiotic. I. Synthesis and chemical properties of cefazolin, ournal of Antibiotics, Volume: 23, Issue: 3, Pages: 131-6, 1970
- Kariyone, Kazuo; Harada, Hirokichi; Karita, Masaru; Yazawa, Hisatoyo, Preparation Of 7-Amino-Cephalo-Sporanic Acid Derivatives, Fujisawa Pharmaceutical Co., Ltd., Japan, GB1336155A, November 7, 1973
- Staiger, D. B.; Warren, R. J.; Zarembo, J. E.; Post, A., NMR spectroscopic analysis of 2-mercapto-5-methyl-1,3,4-thiadiazole in cefazolin, Journal of Pharmaceutical Sciences, Volume: 64, Issue: 8, Pages: 1396-7, 1975
Frequently Asked Questions
Why is it essential to control Cefazolin impurities?
Controlling impurities in Cefazolin is essential because it can affect the safety and efficacy of the drug. They can also cause adverse reactions in patients.
How can Cefazolin impurities be identified?
Impurities in Cefazolin are identified through various analytical techniques, such as high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS).
Which solvent helps analyze Cefazolin impurities?
DMSO, TFA, and water are different types of solvents used in the analysis of impurities in Cefazolin.
What are the temperature conditions required to store Cefazolin impurities?
Cefazolin impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.