Carmustine
General Information
Carmustine Impurities and Carmustine
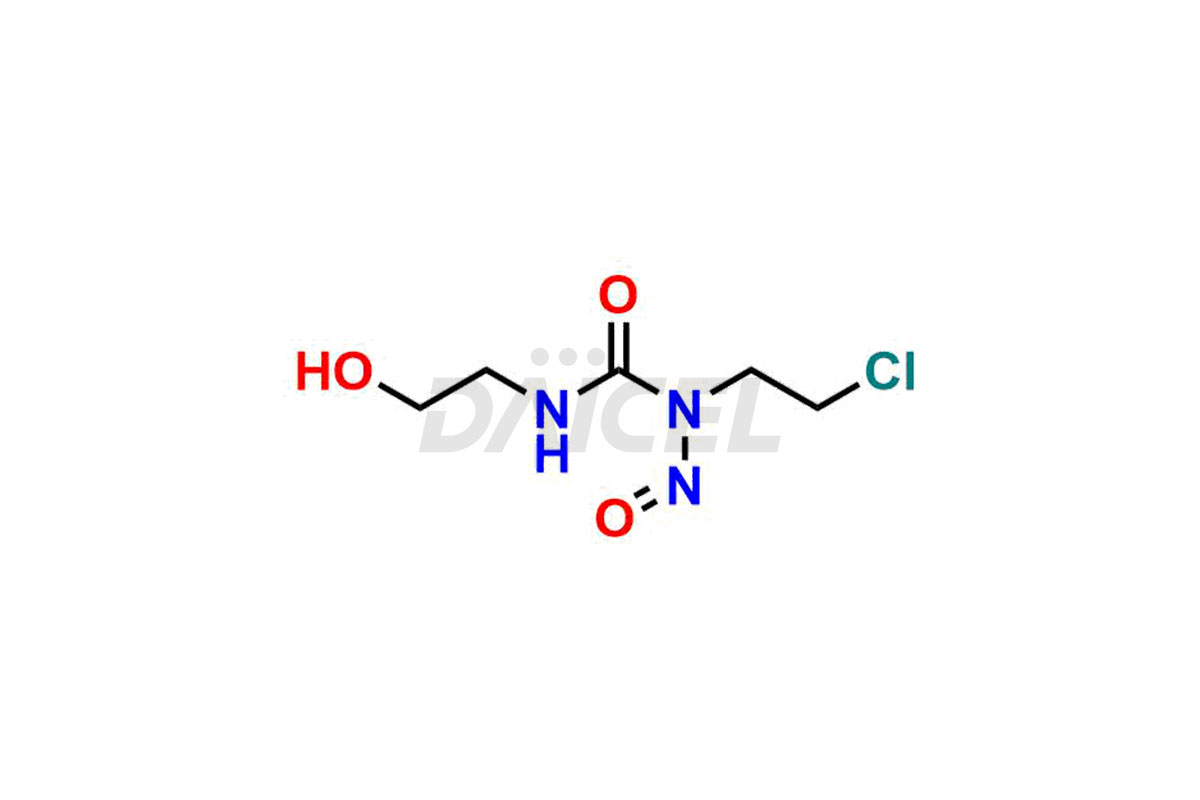
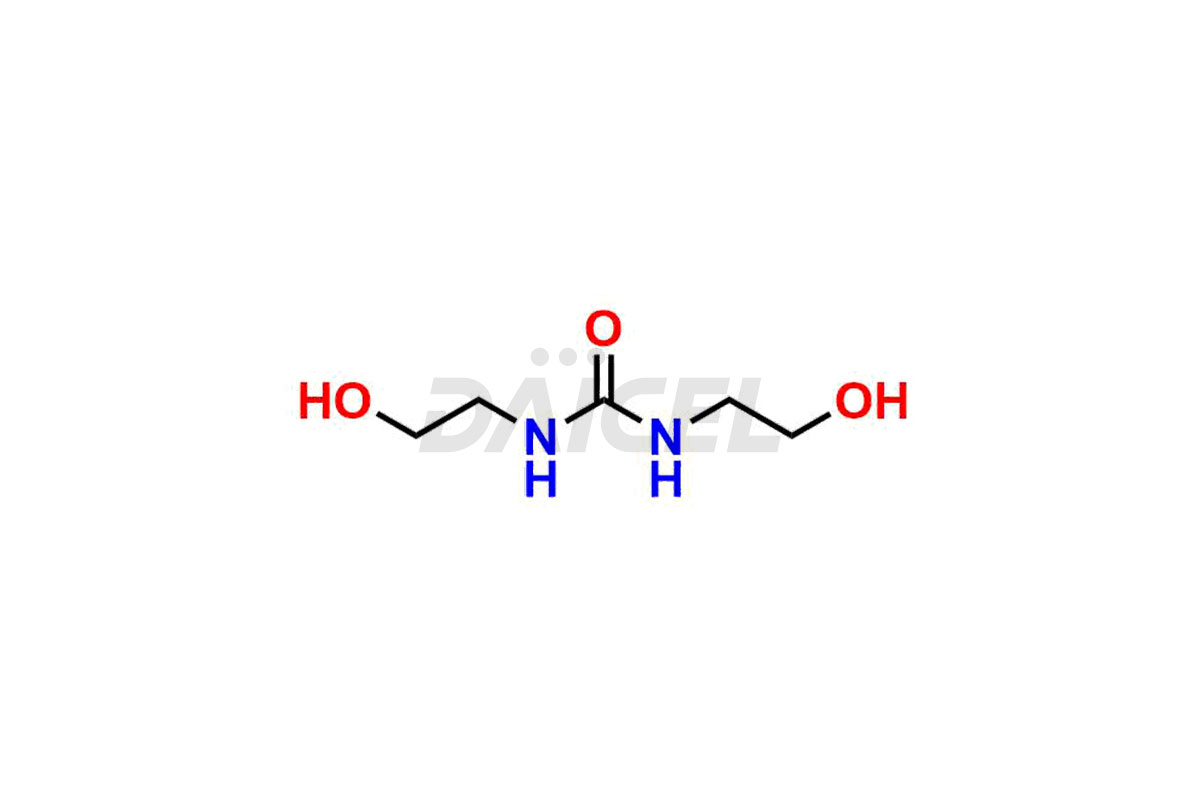
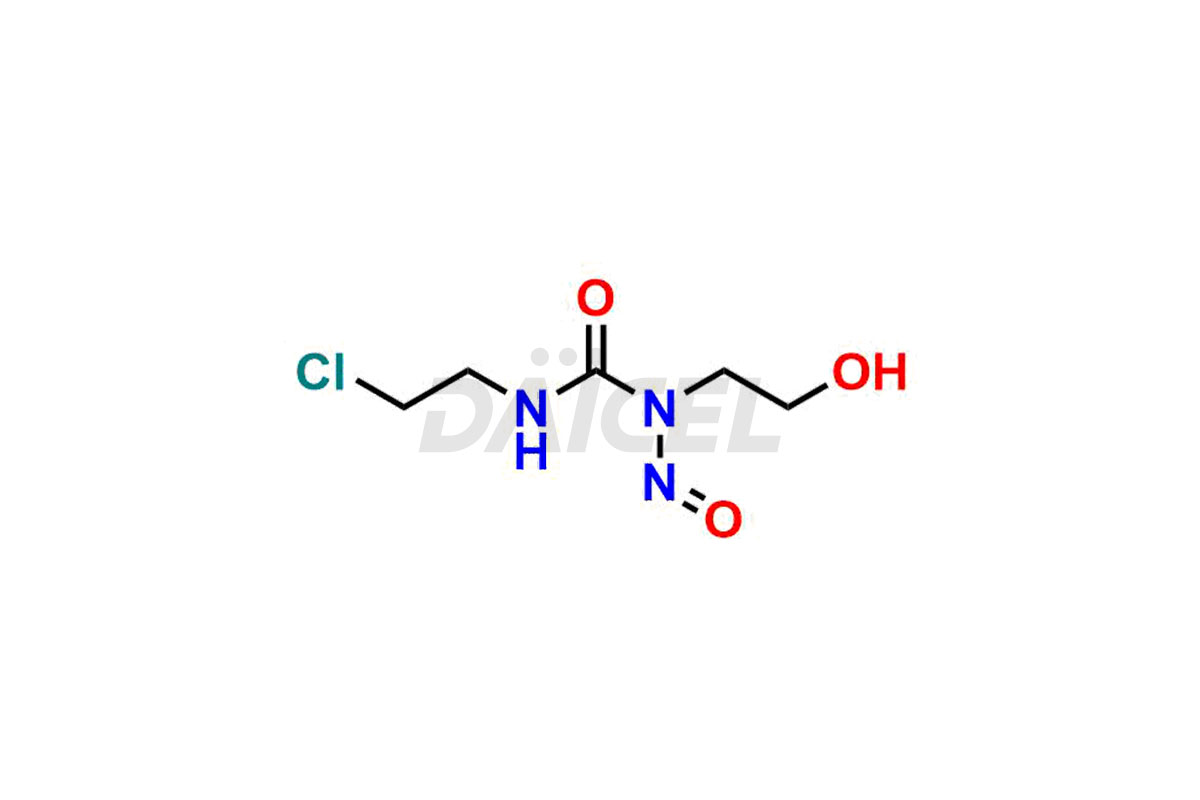
Daicel Pharma synthesizes Carmustine impurities of exceptional quality, such as 1-(2-chloroethyl)-3-(2-hydroxyethyl)-1-nitrosourea, 1,3-bis(2-hydroxyethyl)urea and 3-(2-chloroethyl)-1-(2-hydroxyethyl)-1-nitrosourea. These impurities are crucial to assess the purity, reliability, and safety of Carmustine, an active pharmaceutical ingredient. Besides, Daicel Pharma provides a custom synthesis of Carmustine impurities to meet clients’ demands for delivery worldwide.
Carmustine [CAS: 154-93-8] is an antineoplastic nitrosourea that treats various cancers. As an alkylating agent, it treats leukemias, lymphomas, and breast, testicular, ovarian, gastric, and pancreatic cancer.
Carmustine: Use and Commercial Availability
Carmustine is a medication that can be used alone or in combination with other drugs to treat various types of cancer, including certain brain tumors, Hodgkin and non-Hodgkin lymphomas, and multiple myeloma. Bicnu and Gliadel are the brand names under which Carmustine is available.
Carmustine Structure and Mechanism of Action 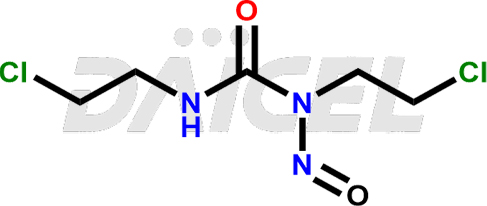
The chemical name of Carmustine is N, N′-Bis(2-chloroethyl)-N-nitrosourea. Its chemical formula is C5H9Cl2N3O2, and its molecular weight is approximately 214.05 g/mol.
Carmustine inhibits enzymatic processes by carbamoylation of amino acids in proteins.
Carmustine Impurities and Synthesis
Carmustine is a chemotherapeutic agent that can form impurities during manufacturing1 or storage. They generate due to degradation, oxidation, or contaminants in the raw materials used for manufacturing. The presence of impurities in Carmustine can impact the efficacy and safety of the drug. So, it is essential to control and monitor them during the manufacturing and storage of Carmustine. It is through rigorous quality control measures, including analytical testing, and monitors the manufacturing process.
Daicel Pharma offers a Certificate of Analysis (CoA) for Carmustine impurity standards, such as 1-(2-chloroethyl)-3-(2-hydroxyethyl)-1-nitrosourea, 1,3-bis(2-hydroxyethyl) urea and 3-(2-chloroethyl)-1-(2-hydroxyethyl)-1-nitrosourea, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we can provide additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Carmustine impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Yanko, William H.; Sharp, Donald E., Process Of Preparing 1,3-Bis(2-Chloroethyl)-1- Nitrosourea, United States Dept. Of Health, Education, and Welfare, United States, CA1082223A1, July 22, 1980
- Yeager, R. L.; Oldfield, E. H.; Chatterji, D. C., Quantitation of 1,3-bis(2-chloroethyl)-1-nitrosourea in plasma using high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 305, Issue: 2, Pages: 496-501, 1984
Frequently Asked Questions
What is the impact of N-nitrosourea-related impurities on Carmustine?
N-nitrosourea-related impurities in Carmustine are potential carcinogens and mutagens which can pose a risk to patient health and needs proper control.
What is the role of impurity profiling in Carmustine?
Impurity profiling in Carmustine helps identify, isolate, and quantify impurities, which can aid in developing strategies to minimize impurity formation and control impurity levels.
Which solvent helps in the analysis of Carmustine impurities?
Acetonitrile or DMSO are the solvents used in analyzing many impurities in Carmustine.
What are the temperature conditions required to store Carmustine impurities?
Carmustine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.