LOAD MORE
You're viewed 9 of 22 products
Daicel Pharma synthesizes Bupropion impurities of exceptional quality, such as (3S,5R,6R)- Bupropion Impurity, (3S,5S,6S)- Bupropion Impurity, 1-(3-chlorophenyl)-2-hydroxypropan-1-one, and so on. These impurities are crucial to assess the purity, reliability, and safety of Bupropion, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Bupropion impurities to meet clients’ demands for worldwide delivery.
Bupropion [CAS: 34911-55-2] is an aminoketone antidepressant that treats depression and helps patients quit smoking. It belongs to a novel class of antidepressants that differs structurally from most first-generation tricyclics and second-generation SSRI antidepressants. It treats excessive daytime sleepiness (EDS) associated with narcolepsy.
Bupropion, an antidepressant drug, also helps in smoking cessation. It treats the major depressive disorder. Further, it treats EDS associated with narcolepsy. Some of the brand names under which Bupropion is available as Aplenzin, Forfivo Xl, Wellbutrin, and Zyban.
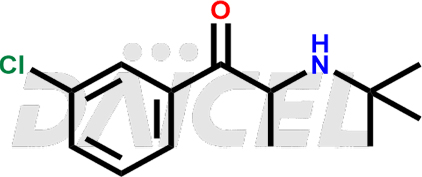
The chemical name of Bupropion is 2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one. Its chemical formula is C13H18ClNO, and its molecular weight is approximately 239.74 g/mol.
The mechanism of action of Bupropion is unknown.
Impurities in Bupropion form during its synthesis1, storage, and transportation affect the drug’s purity, stability, efficacy, and safety. Common impurities include related compounds, residual solvents, and degradation products. These impurities can cause toxic and carcinogenic effects. So, it is necessary to control them in Bupropion through strict quality control measures and analytical methods during their manufacturing, storage, and distribution to ensure their safety and efficacy for patients.
Daicel Pharma offers a Certificate of Analysis (CoA) for Bupropion impurity standards, such as (3S,5R,6R)- Bupropion Impurity, (3S,5S,6S)- Bupropion Impurity, 1-(3-chlorophenyl)-2-hydroxypropan-1-one, and so on, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. Furthermore, on request, we can provide additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Bupropion impurities or degradation products and labeled compounds to assess the effectiveness of generic Bupropion. We also offer Bupropion Hydrochloride-D9, Erythro-Dihydro Bupropion.HCl – D9, Hydroxy Bupropion-D6, and Threo-Dihydro Bupropion.HCl – D9, deuterium-labeled Bupropion compounds useful in bio-analytical research, such as BA/BE studies. A complete characterization report accompanies every delivery.
Impurity testing should be conducted at regular intervals during drug development, including during the synthesis of the API, production of drug products, and stability studies. The testing frequency depends on the regulatory requirements and the risk associated with the impurities.
Yes, impurities in Bupropion can affect the drug efficacy by reducing the potency of the API or interfering with its mechanism of action.
The detection of impurities in Bupropion is through analytical methods such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), etc.
Bupropion impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.