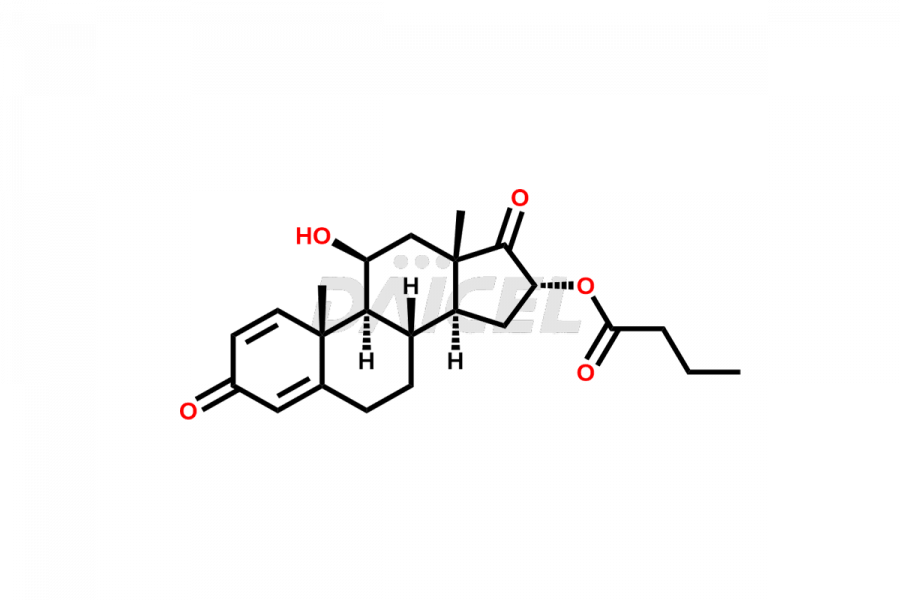
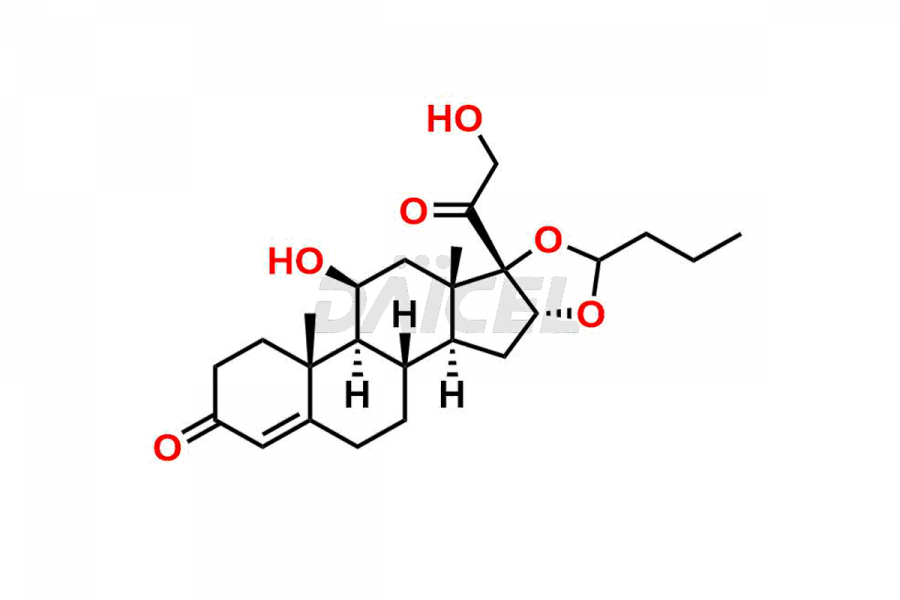
Budesonide
General Information
Budesonide Impurities and Budesonide
Daicel Pharma synthesizes Budesonide impurities of exceptional quality, such as Budesonide 17-keto Impurity and Budesonide Related Compound G. These impurities are crucial to assess the purity, reliability, and safety of Budesonide, an active pharmaceutical ingredient. Besides, Daicel Pharma provides custom synthesis of Budesonide impurities to meet clients’ demands for worldwide delivery.
Budesonide [CAS: 51333-22-3] is a synthetic glucocorticoid. It has properties that can reduce inflammation and help regulate the immune system. Budesonide reduces inflammation and acts as a bronchodilator agent.
Budesonide: Use and Commercial Availability
Budesonide is a corticosteroid medication used to manage and treat inflammatory diseases that affect the airways and gastrointestinal tract. It treats allergic rhinitis, upper respiratory allergies, asthma, chronic obstructive pulmonary disease (COPD), Crohn’s disease, and ulcerative colitis. The dosage and administration of Budesonide vary depending on the condition, the patient’s age, and prior therapy. It is available in various forms, including nasal sprays, inhalation suspensions, metered-dose inhalers, inhalation powders, and rectal foams. Budesonide is available under various brands such as Uceris, Tarpeyo, Rhinocort, Pulmicort, Ortikos, Entocort Ec, and more.
Budesonide Structure and Mechanism of Action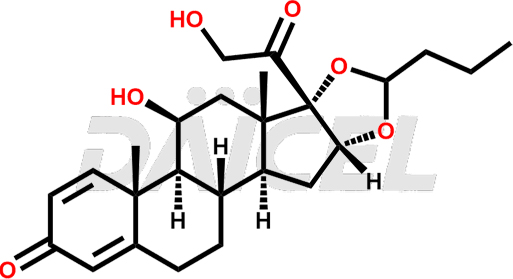
The chemical name of Budesonide is (11β,16α)-16,17-[Butylidenebis(oxy)]-11,21-dihydroxypregna-1,4-diene-3,20-dione. Its chemical formula is C25H34O6, and its molecular weight is approximately 430.5 g/mol.
Budesonide is a corticosteroid that possesses glucocorticoid activity. It binds to glucocorticoid receptors and causes anti-inflammatory effects.
Budesonide Impurities and Synthesis
During the manufacturing1 of Budesonide, impurities may form and affect the safety and efficacy of the drug. They form during synthesis, degradation, and storage conditions. It is necessary to control and monitor impurity levels to ensure the quality and safety of the drug. Regulatory agencies, like the US FDA, have established guidelines and limits for these impurities.
Daicel Pharma offers a Certificate of Analysis (CoA) for Budesonide impurity standards, such as Budesonide 17-keto Impurity and Budesonide Related Compound G, generated from an analytical facility compliant with cGMP standards. The CoA includes a comprehensive characterization report comprising data from techniques like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Furthermore, on request, we can provide additional data like 13C-DEPT and CHN. Daicel Pharma can synthesize unknown Budesonide impurities or degradation products. A complete characterization report accompanies every delivery.
References
FAQ's
References
- Brattsand, Ralph L.; Ekenstam, Bo T. af; Claeson, Karl G.; Thalen, Bror A., Steroids, Processes For Their Manufacture And Preparations Containing Same, Aktiebolag Bofors, US3929768A, December 30, 1975
- Faouzi, M. A.; Dine, T.; Luyckx, M.; Brunet, C.; Gressier, B.; Cazin, M.; Wallaert, B.; Cazin, J. C., High-performance liquid chromatographic method for the determination of budesonide in bronchoalveolar lavage of asthmatic patients, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 664, Issue: 2, Pages: 463-7,1995
Frequently Asked Questions
How can the formation of impurities in Budesonide be prevented?
The formation of Budesonide impurities can be prevented through diligent process development and optimization, the use of high-quality starting materials, and the implementation of appropriate storage and transportation conditions.
What are the common Budesonide impurities?
Common Budesonide impurities include diastereomers, degradants, and residual solvents.
Can impurities affect the stability of Budesonide?
Yes, Budesonide impurities can affect the drug's stability, and reduce potency, decrease shelf life, and potential degradation.
What are the temperature conditions required to store Budesonide impurities?
Budesonide impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.