LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma is a reliable source for synthesizing high-quality Brimonidine impurities, specifically 5-Bromo-6-isothiocyanato quinoxaline, Brimonidine Carbamothioate Impurity, Brimonidine Cyclic Impurity, Brimonidine Impurity-A, B, C, D, E, & H, Brimonidine Unknown Impurity I, Brimonidine Unknown Impurity II, and O-Isopropyl(5-Bromoquinoxalin-6-yl)Carbamothioate. These impurities play a crucial role in assessing the quality, stability, and safety of the active pharmaceutical ingredient, Brimonidine. Daicel Pharma also offers custom synthesis of Brimonidine impurities, which can be shipped worldwide.
Brimonidine [CAS: 59803-98-4] is a medicine to treat open-angle glaucoma or ocular hypertension. It is an imidazole derivative and selectively activates alpha-2 adrenergic receptors in the eye. Brimonidine is also a quinoxaline derivative and a secondary amine. It is known to have antihypertensive properties and acts as an adrenergic agonist, specifically an alpha-adrenergic agonist.
Brimonidine administration is as eye drops to treat open-angle glaucoma or ocular hypertension, either alone or in combination with brinzolamide. It reduces intraocular pressure through the selective activation of alpha-2 adrenergic receptors. Additionally, it treats persistent facial erythema of rosacea in adults. Brimonidine is available under various brand names, including Alphagan, Combigan, Lumify, Mirvaso, Qoliana, Simbrinza, etc.
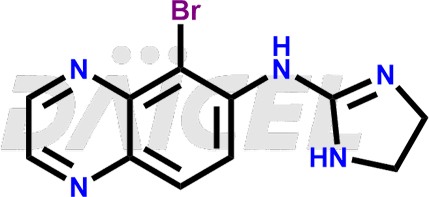
The chemical name of Brimonidine is 5-Bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine. Its chemical formula is C11H10BrN5, and its molecular weight is approximately 292.13 g/mol.
Brimonidine acts as an agonist at alpha-2 adrenoceptors and lowers intraocular pressure. It causes the blood vessels to narrow, reducing the aqueous humor production and increasing its outflow leading to a reduction in intraocular pressure.
During the synthesis1 of Brimonidine, impurities may form, which can affect drug safety and efficacy. Therefore, it is necessary to identify and control the impurities to ensure the quality of the final product. The impurities form due to various factors such as contaminants in the starting materials, process impurities, and degradation products. Manufacturers must monitor and control them during the entire manufacturing process and establish appropriate specifications to ensure the safety and efficacy of the drug.
Daicel Pharma issues a Certificate of Analysis (CoA) for Brimonidine impurity standards, including 5-Bromo-6-isothiocyanato quinoxaline, Brimonidine Carbamothioate Impurity, Brimonidine Cyclic Impurity, Brimonidine Impurity-A, B, C, D, E, & H, Brimonidine Unknown Impurity I, Brimonidine Unknown Impurity II and O-Isopropyl(5-Bromoquinoxalin-6-yl)Carbamothioate. The CoA is from an analytical facility that complies with current Good Manufacturing Practices (cGMP) and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS2, and HPLC purity. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can prepare unknown Brimonidine impurities or degradation products. A complete characterization report accompanies each delivery.
The risks of Brimonidine impurities can include decreased efficacy, increased toxicity, and harm to patients' safety and health.
Impurities in Brimonidine affect the drug bioavailability by interfering with drug absorption or metabolism, leading to decreased efficacy or toxicity.
Impurities in Brimonidine are detected through various analytical techniques, such as HPLC and LC-MS.
Brimonidine impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.