LOAD MORE
You're viewed 9 of 17 products
Daicel Pharma synthesizes high-quality Bosentan impurities, including Bosentan Dimer Impurity, Bosentan Impurity-2, Bosentan Impurity-3, Bosentan USP Related Compound-B, N,N′-[5-(2-Methoxyphenoxy)[2,2′-bipyrimidine]-4,6-diyl]bis[4(1,1dimethylethyl)benzenesulfonamide], Bosentan Impurity-4, and so on. These impurities are essential for evaluating the quality, stability, and safety of an active pharmaceutical ingredient, Bosentan. Additionally, Daicel Pharma offers a customized synthesis of Bosentan impurities for delivery globally to meet the specific needs of our customers.
Bosentan [CAS: 147536-97-8] is a pyrimidine derivative that is an endothelin receptor antagonist and antihypertensive agent. It treats pulmonary arterial hypertension (PAH).
Pulmonary artery hypertension is a condition that can cause considerable morbidity and mortality worldwide. The primary treatment for this condition is with an endothelin-1 antagonist such as Bosentan. Bosentan is an FDA-approved medication that manages and treats pulmonary artery hypertension in patients with significant physical limitations to improve their exercise capacity and reduce the rate of clinical worsening. The medicine is available under the tradename Tracleer.
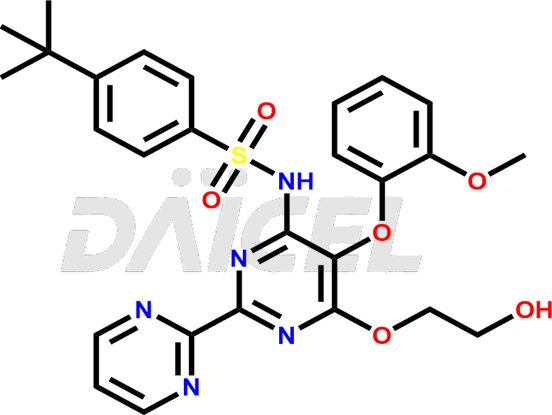
The chemical name of Bosentan is 4-(1,1-Dimethylethyl)-N-[6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)[2,2′-bipyrimidin]-4-yl]benzenesulfonamide. Its chemical formula is C27H29N5O6S, and its molecular weight is approximately 551.6 g/mol.
Bosentan is an endothelin receptor antagonist in lung tissue and relaxes the vascular smooth muscle.
As with any drug, impurities can form during the manufacturing1 process of Bosentan. These impurities can be due to starting materials, synthetic intermediates, or degradation products. Some of them may be toxic or affect the drug efficacy, so it is necessary to control their formation and limit their presence in the final product. Analytical methods, such as high-performance liquid chromatography (HPLC), help detect and quantify these impurities in Bosentan batches and set specifications to ensure that impurity levels are within acceptable limits. Their control is essential to maintain the drug’s safety, quality, and efficacy.
Daicel Pharma offers a Certificate of Analysis (CoA) for Bosentan impurity standards, which includes Bosentan Dimer Impurity, Bosentan Impurity-2, Bosentan Impurity-3, Bosentan USP Related Compound-B, N,N′-[5-(2-Methoxyphenoxy)[2,2′-bipyrimidine]-4,6-diyl]bis[4(1,1dimethylethyl) benzenesulfonamide], Bosentan Impurity-4, and so on. The CoA is produced from a cGMP-compliant analytical facility and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS2, and HPLC purity. Additional characterization data, such as 13C-DEPT and CHN, can also be provided on request. Daicel Pharma can create unknown Bosentan impurities or degradation products and supply labeled compounds to evaluate the effectiveness of Bosentan. Additionally, Daicel Pharma offers Bosentan– D4, deuterium-labeled Bosentan standard useful in bio-analytical research, such as BA/BE studies. Each delivery has a complete characterization report.
Bosentan impurities form during manufacturing due to various factors, such as impurities in starting materials, intermediates, reagents, and purification steps.
Bosentan impurities are identified and characterized using various analytical methods, such as HPLC and LC-MS. Mass spectrometry can also help determine the molecular weight of impurities.
Yes, impurities in Bosentan can change over time due to storage conditions and exposure to light and heat. It is vital to monitor the quality of Bosentan throughout its shelf life to ensure that it remains safe and effective.
Bosentan impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.