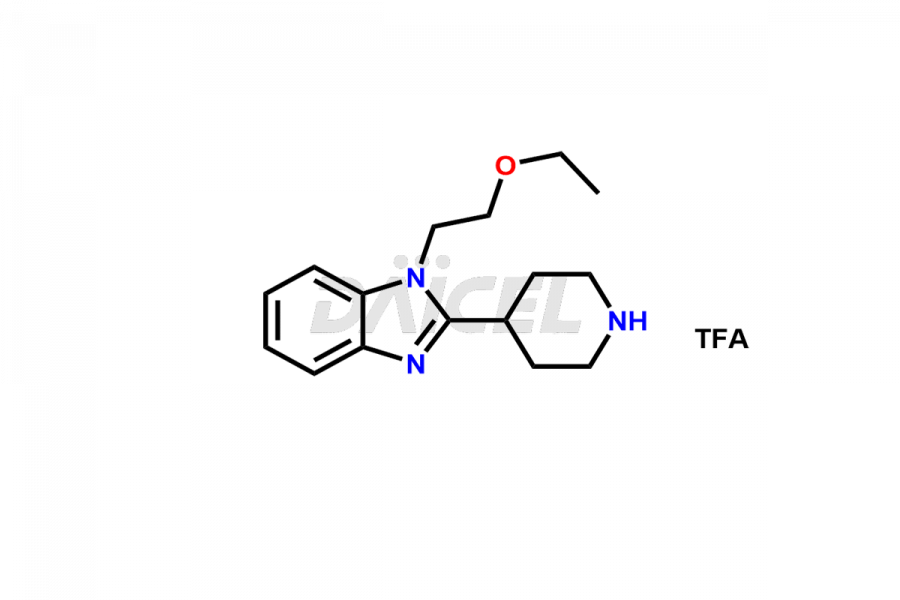
Bilastine
General Information
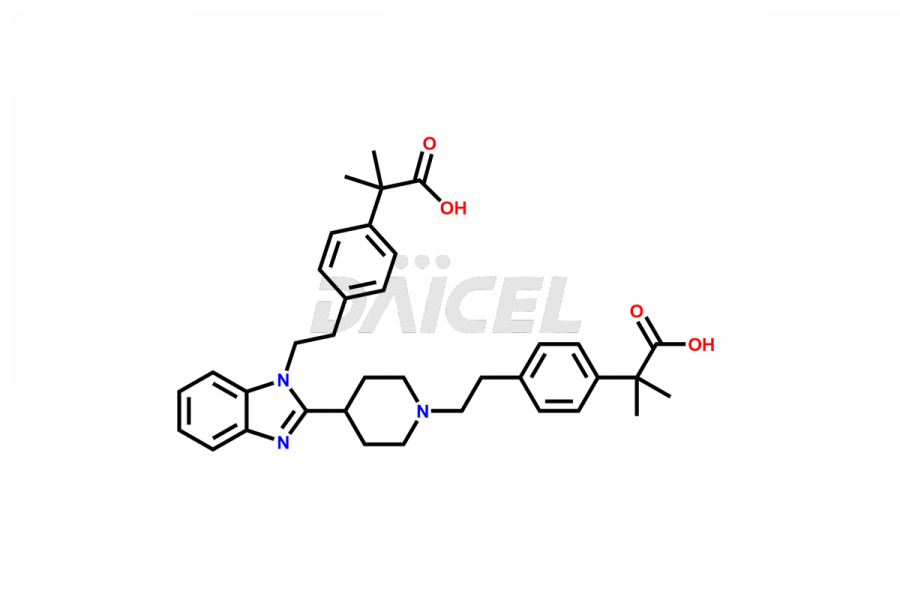
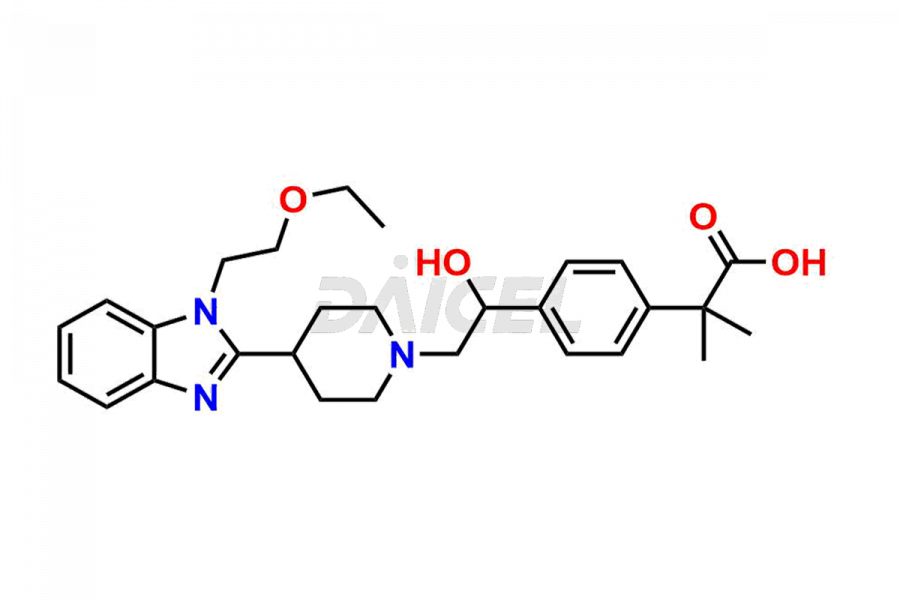
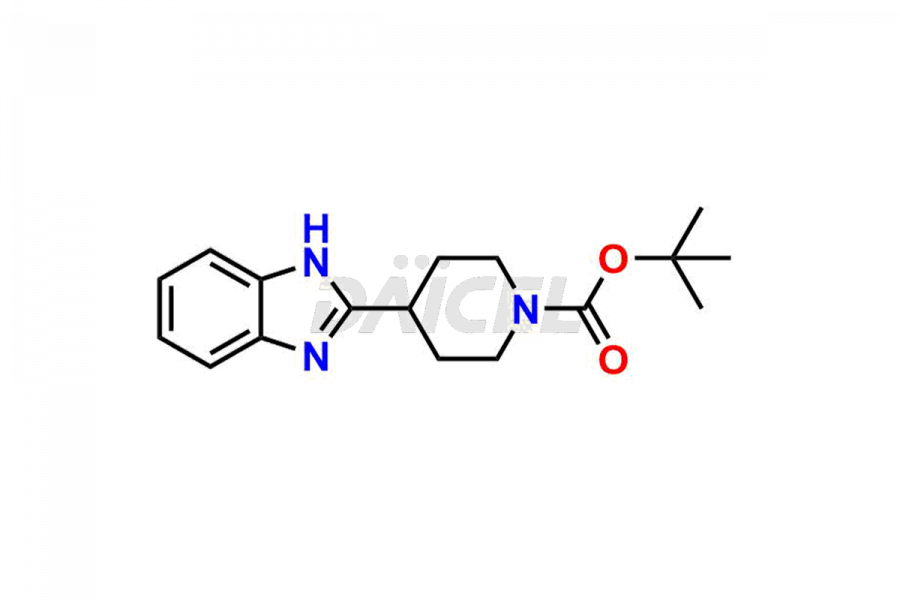
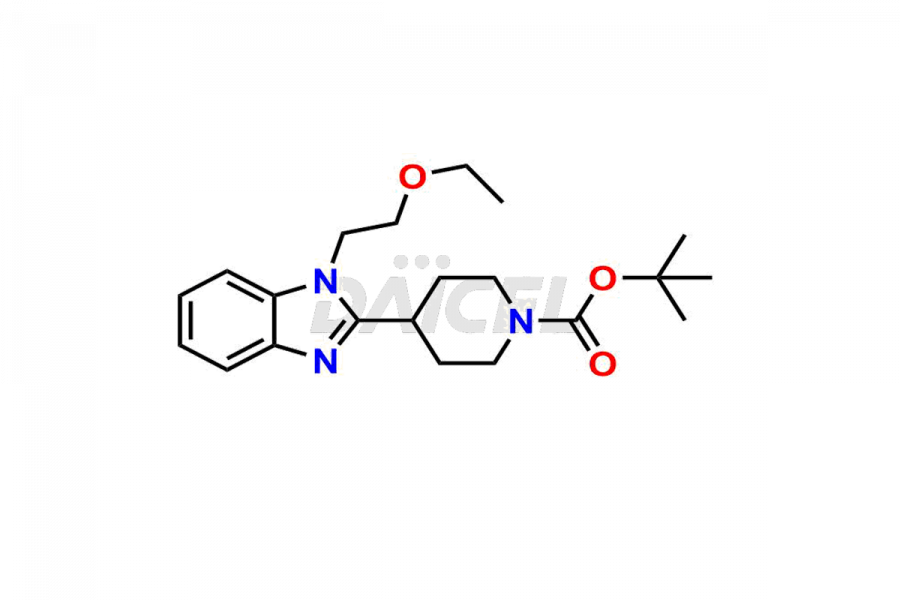
Bilastine Impurities and Bilastine
Daicel Pharma synthesizes high-quality Bilastine impurities, including 1′-Hydroxy Bilastine, Bilastine Impurity 1, Bilastine Impurity 2, Bilastine Impurity 7, and Bilastine N-Oxide Impurity. These impurities are essential for evaluating the quality, stability, and safety of Bilastine, an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Bilastine impurities for delivery globally to meet the specific needs of our customers.
Bilastine [CAS: 202189-78-4] is an antihistamine that exhibits high selectivity for the H1 histamine receptor antagonist, providing rapid onset of action and long-lasting effects. This medication belongs to the benzimidazole class. Bilastine treats allergic rhinitis and urticaria associated with excessive histamine release.
Bilastine: Use and Commercial Availability
Bilastine is a highly effective, non-sedating, second-generation, H1 receptor antihistamine treating allergic rhinoconjunctivitis and chronic urticaria in patients. Bilastine is available under various brands such as Allertine, Bilargo, Blexten, Bilaxten, Clatra, Ilaxten, etc.
Bilastine Structure and Mechanism of Action 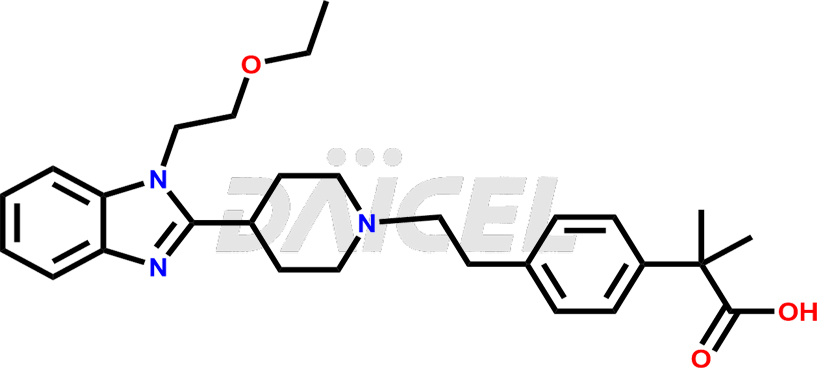
The chemical name of Bilastine is 4-[2-[4-[1-(2-Ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidinyl]ethyl]-α,α-dimethylbenzeneacetic acid. Its chemical formula is C28H37N3O3, and its molecular weight is approximately 463.6 g/mol.
Bilastine prevents the activation of H1 receptors and releases histamine from mast cells, thus reducing allergic symptoms.
Bilastine Impurities and Synthesis
During the manufacturing1 of Bilastine, impurities may form due to factors such as impure starting materials, reagents, or processing conditions. These impurities can affect the safety and efficacy of the drug. So it is essential to control them within acceptable limits during its manufacturing. Quality control measures, such as analytical testing and process optimization, are necessary to ensure the purity and quality of Bilastine for safe and effective use in patients.
Daicel Pharma provides a Certificate of Analysis (CoA) for Bilastine impurity standards, including 1′-Hydroxy Bilastine, Bilastine Impurity 1, Bilastine Impurity 2, Bilastine Impurity 7, and Bilastine N-Oxide Impurity. The CoA is generated from a cGMP-compliant analytical facility and includes comprehensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. We can also give additional characterization data like 13C-DEPT and CHN on request. Daicel Pharma is capable of creating unknown Bilastine impurities or degradation products. Each delivery has a complete characterization report.
References
FAQ's
References
- Orjales, Aurelio; Rubio, Victor; Bordell, Maravillas, Benzimidazole derivatives with antihistaminic activity, Fabrica Espanola de Productos Quimicos y Farmaceuticos, S.A. (Faes), Spain, EP818454B1, April 14, 2004 (https://patents.google.com/patent/EP0818454B1/en)
- Chowdary, V. Amarendra; Kota, Anusha; Muneer, Syed, Method development and validation of new RP-HPLC method for the estimation of Bilastine in pharmaceutical dosage form, World Journal of Pharmacy and Pharmaceutical Sciences, Volume: 6, Issue: 8, Pages: 2297-2315, 2017
- Katta, Rambabu; Murty, N. N. V. V. S. S. Narayana; Ramasrinivas; Rao, G. N., Stability indicating method development and validation for the determination of bilastine and its impurities by UPLC method, International Journal of Pharmaceutical Sciences and Research, Volume: 11, Issue: 3, Pages: 1312-1321, 2020
Frequently Asked Questions
What are the potential health risks associated with Bilastine impurities?
The potential health risks associated with Bilastine impurities include toxicological effects, allergic reactions, and reduced efficacy of the drug product. It is vital to control and minimize the impurity levels in Bilastine.
What are the challenges associated with controlling Bilastine impurities?
The challenges associated with controlling Bilastine impurities include the complex synthetic process, impurity introduction from various sources, and managing manufacturing efficiency.
Which solvent helps in the analysis of Bilastine impurities?
Methanol is a solvent used in analyzing many impurities in Bilastine.
What are the temperature conditions required to store Bilastine impurities?
Bilastine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.