Bepotastine
General Information
Bepotastine Impurities and Bepotastine
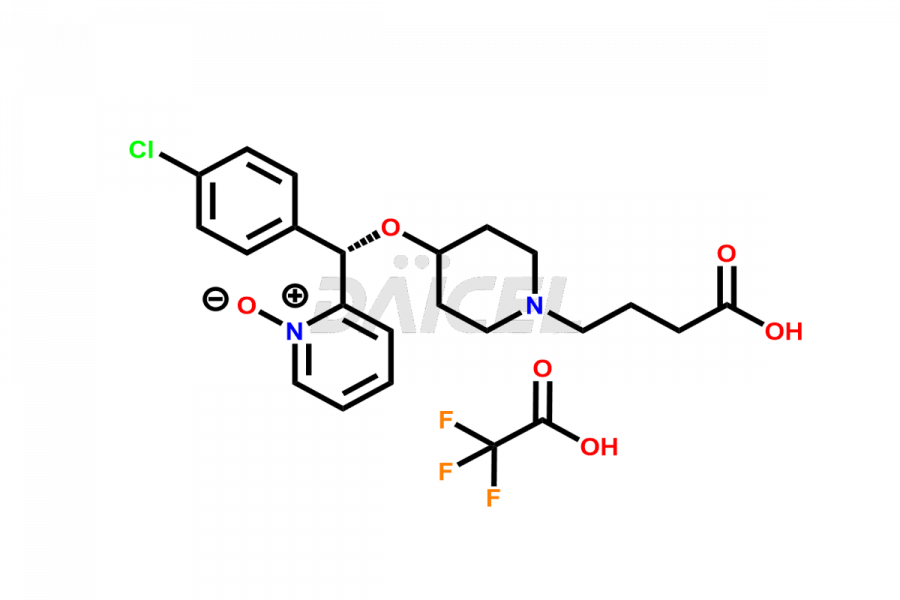
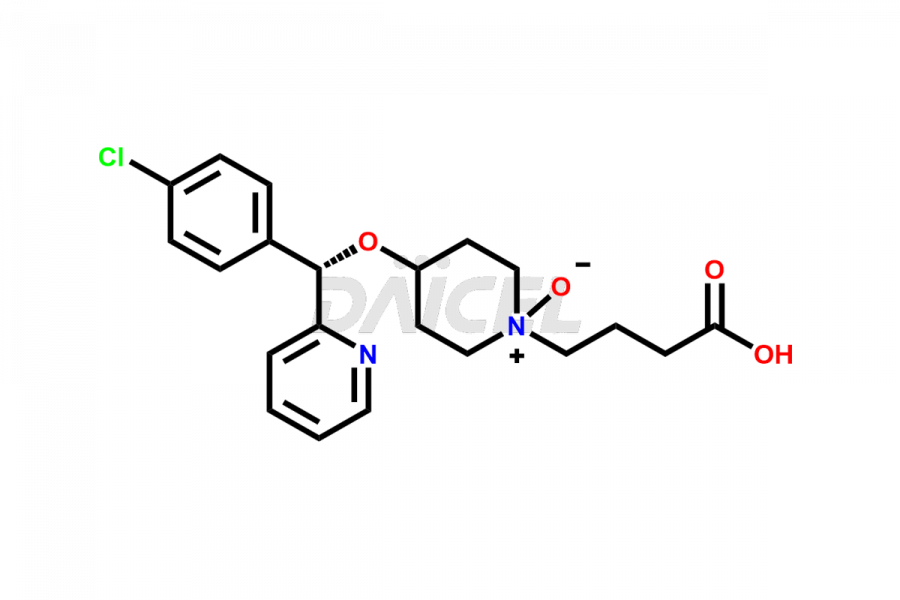
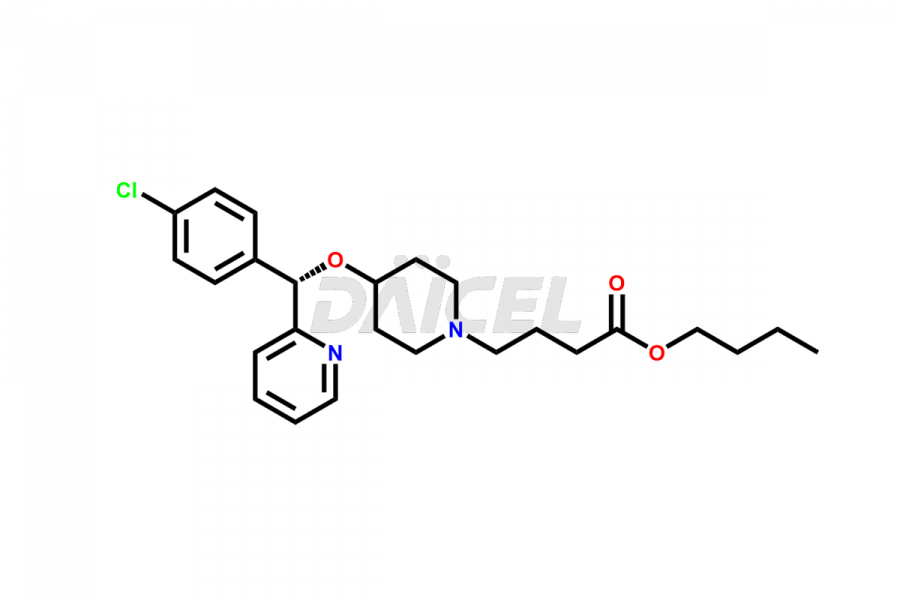
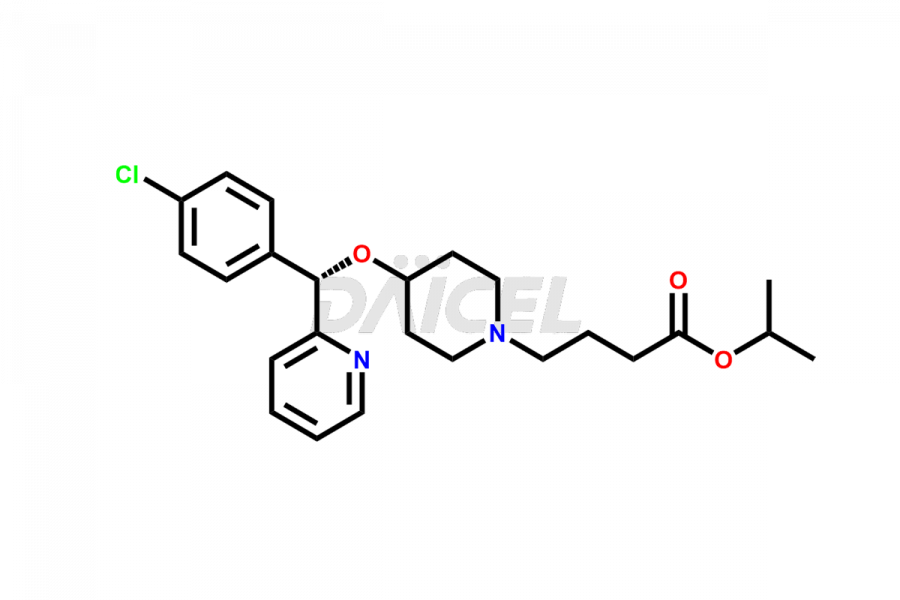
Daicel Pharma synthesizes high-quality Bepotastine impurities, including Bepotastine N-Oxide (trifluoroacetate salt), Bepotastine piperidine N-Oxide (Mixture of diastereomers), SBPB (Bepotastine n-Butyl Ester), and SBPI (Bepotastine Isopropyl Ester). These impurities are essential for evaluating the quality, stability, and safety of Bepotastine, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Bepotastine impurities for global delivery to meet the specific needs of our customers.
Bepotastine [CAS: 125602-71-3] is a non-sedating, selective histamine (H1) receptor antagonist with topical application. Its benzenesulfonate salt treats itching related to allergic conjunctivitis.
Bepotastine: Use and Commercial Availability
Bepotastine is an ophthalmic antihistamine that has additional anti-inflammatory and mast cell stabilizing properties. It can inhibit eosinophilic infiltration, IL-5 production, and leukotriene B4 and D4 activity. The drug treats allergic rhinitis and pruritus/itching linked to urticaria and other skin conditions. Bepreve is the trade name under which Bepotastine is available.
Bepotastine Structure and Mechanism of Action 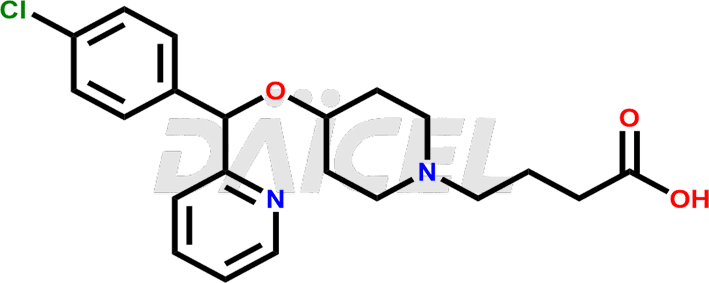
The chemical name of Bepotastine is 4-[(S)-(4-Chlorophenyl)-2-pyridinylmethoxy]-1-piperidinebutanoic acid. Its chemical formula is C21H25ClN2O3, and its molecular weight is approximately 388.9 g/mol.
Bepotastine inhibits the release of histamine from mast cells.
Bepotastine Impurities and Synthesis
During the manufacturing1 of Bepotastine, impurities generate due to various factors such as the starting materials, chemical reactions, and processing conditions. These impurities can affect the drug quality, safety, and efficacy. So, it is necessary to control them to ensure their levels are within acceptable limits. The identification and quantification of impurities, as well as the establishment of appropriate specifications and testing methods, are crucial for ensuring the quality of Bepotastine and minimizing potential risks to patients.
Daicel Pharma provides a Certificate of Analysis (CoA) for Bepotastine impurity standards, including Bepotastine N-Oxide (trifluoroacetate salt), Bepotastine piperidine N-Oxide (Mixture of diastereomers), SBPB (Bepotastine n-Butyl Ester), and SBPI (Bepotastine Isopropyl Ester). The CoA is generated from a cGMP-compliant analytical facility and includes comprehensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We can also give additional characterization data like 13C-DEPT and CHN upon request. Daicel Pharma is capable of creating unknown Bepotastine impurities or degradation products. Each delivery has a complete characterization report.
References
FAQ's
References
- Kita, Jun-ichiro; Fujiwara, Hiroshi; Takamura, Shinji; Yoshioka, Ryuzo; Ozaki, Yauhiko; Yamada, Shin-ichi, Acid-Addition Salts Of Optically Active Piperidine Compound And Process For Producing The Same, Ube Industries, Ltd., Japan,Tanabe Seiyaku Co., Ltd., Japan, EP949260B1. May22, 2002
- Kotla et al, Stability indicating HPLC method for the quantification of bepotastine besilate and its related substances, Der Pharma Chemica 6(2):343-351, April 2014
Frequently Asked Questions
How can the formation of Bepotastine impurities be minimized?
The formation of Bepotastine impurities can be minimized by optimizing the synthetic process, selecting the proper starting materials, and implementing appropriate storage conditions.
How are the Bepotastine impurities identified?
Impurities in Bepotastine are identified through various analytical techniques, such as HPLC and LC-MS, which identify and quantify impurities in the drug substance or product.
Which solvent helps in the analysis of Bepotastine impurities?
Methanol is a solvent used in analyzing many impurities in Bepotastine.
What are the temperature conditions required to store Bepotastine impurities?
Bepotastine impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.