Benfotiamine
General Information
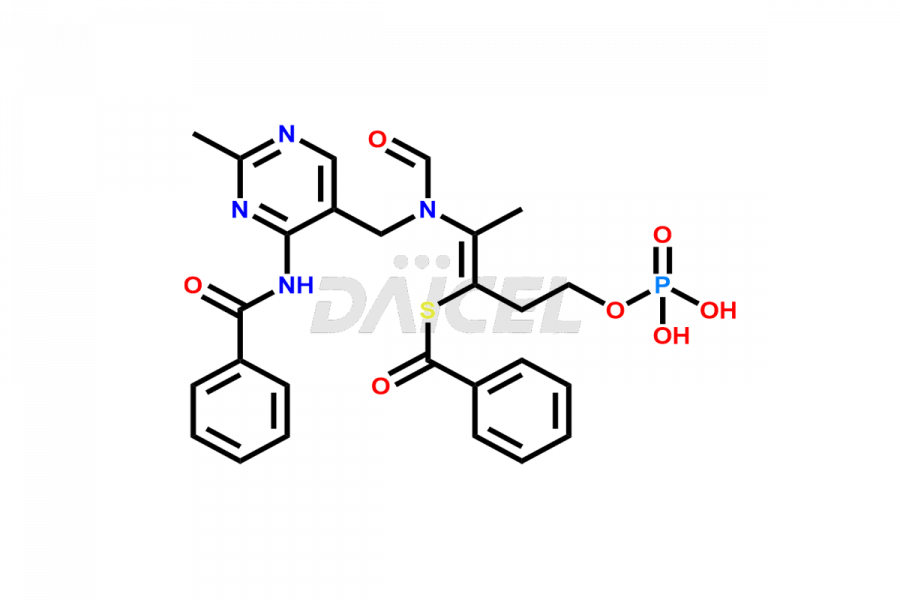
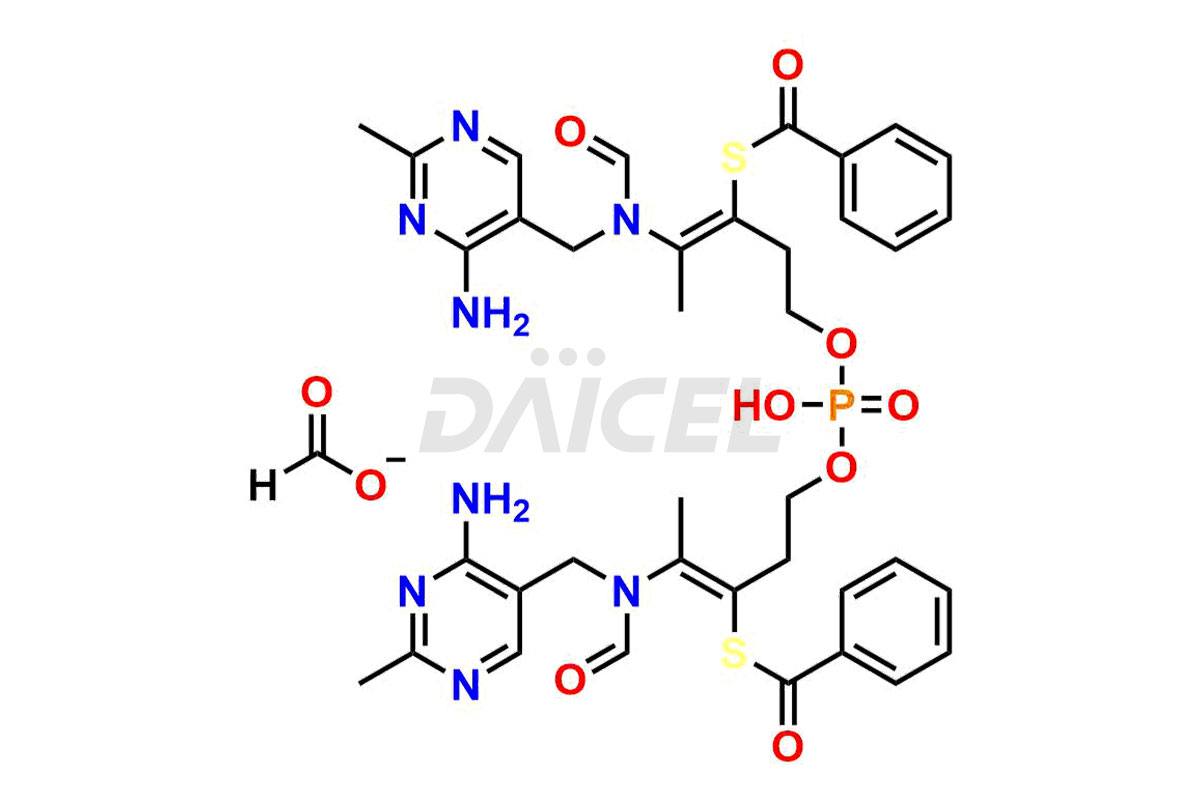
Benfotiamine Impurities and Benfotiamine
Daicel Pharma synthesizes high-quality Benfotiamine impurities, including Benfotiamine Amide Impurity and Di ester base of Benfotiamine Formate salt. These impurities are essential for evaluating the quality, stability, and safety of Benfotiamine, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Benfotiamine impurities for global delivery to meet the specific needs of our customers.
Benfotiamine [CAS: 22457-89-2] is a synthetic thiamine analog (Vitamin B1). It acts as an immunological adjuvant, nutraceutical, antioxidant, provitamin B1, and protective agent. Benfotiamine treats and prevents diabetic nephropathy and type 2 diabetes mellitus.
Benfotiamine: Use and Commercial Availability
Benfotiamine is a vitamin B1 (thiamine) derivative that is fat-soluble and absorbed up to 3.6 times more efficiently than water-soluble forms. Studies have demonstrated that Benfotiamine can significantly improve neuropathy scores, increase nerve conduction velocity, reduce HbA1c levels, and alleviate pain in diabetic patients. It may also help prevent diabetic nephropathy and retinopathy. Benfotiamine is available under various brands, including Absolut-3G, Agein, Benalgis, Benfage, etc.
Benfotiamine Structure and Mechanism of Action 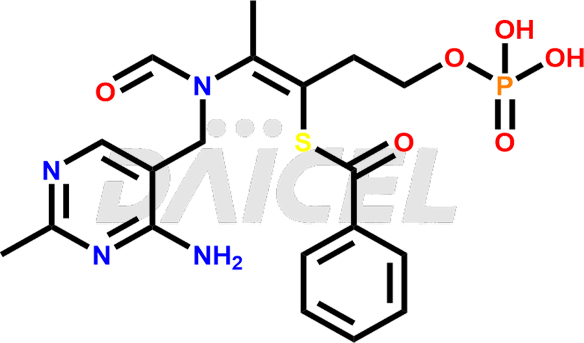
The chemical name of Benfotiamine is S-[2-[[(4-amino-2-methyl-5-pyrimidinyl)methyl]formylamino]-1-[2-(phosphonooxy)ethyl]-1-propen-1-yl] Benzenecarbothioic acid ester. Its chemical formula is C19H23N4O6PS, and its molecular weight is approximately 466.4 g/mol.
Its exact mechanism of action is not known.
Benfotiamine Impurities and Synthesis
The impurities that generate during the synthesis of Benfotiamine include starting materials, by-products, and degradation products. These impurities can harm drug quality, safety, and efficacy. So, it is necessary to control and monitor these impurities during manufacturing and ensure their levels are within acceptable limits according to regulatory guidelines. It is possible by using appropriate analytical methods, process optimization, and quality control measures.
Daicel Pharma provides a Certificate of Analysis (CoA) for Benfotiamine impurity standards, including Benfotiamine Amide Impurity and Di ester base of Benfotiamine Formate salt. The CoA is generated from a cGMP-compliant analytical facility and includes comprehensive characterization data1 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We can also give additional characterization data like 13C-DEPT and CHN on request. Daicel Pharma is capable of creating unknown Benfotiamine impurities or degradation products. Each delivery has a complete characterization report.
References
FAQ's
References
- Vinas, Pilar; Bravo-Bravo, Maria; Lopez-Garcia, Ignacio; Hernandez-Cordoba, Manuel, Determination of benfothiamine in nutraceuticals using dispersive liquid-liquid microextraction coupled to liquid chromatography, Analytical Methods, Volume: 4, Issue: 9, Pages: 2759-2763, 2012
- Nanjeshgowda; Babu, Jose Gnana C.; Mani, Tamizh T., Validated RP-HPLC method for the quantitation of Benfotiamine in bulk and tablet dosage form, International Journal of Pharma Research and Health Sciences, Volume: 5, Issue: 1, Pages: 1558-1562, 2017
Frequently Asked Questions
How are the impurity levels in Benfotiamine measured?
The impurity levels in Benfotiamine can be measured using analytical methods such as high-performance liquid chromatography (HPLC) or liquid chromatography-mass spectrometry (LC-MS).
How does the storage condition affect the impurity profile of Benfotiamine?
Conditions such as temperature, humidity, and light exposure during storage can affect the impurity profile of Benfotiamine, leading to the formation of degradation products and other impurities.
Which solvent helps in the analysis of Benfotiamine impurities?
DMSO is a solvent used in analyzing many impurities in Benfotiamine.
What are the temperature conditions required to store Benfotiamine impurities?
Benfotiamine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.