Baricitinib
General Information
Baricitinib Impurities and Baricitinib
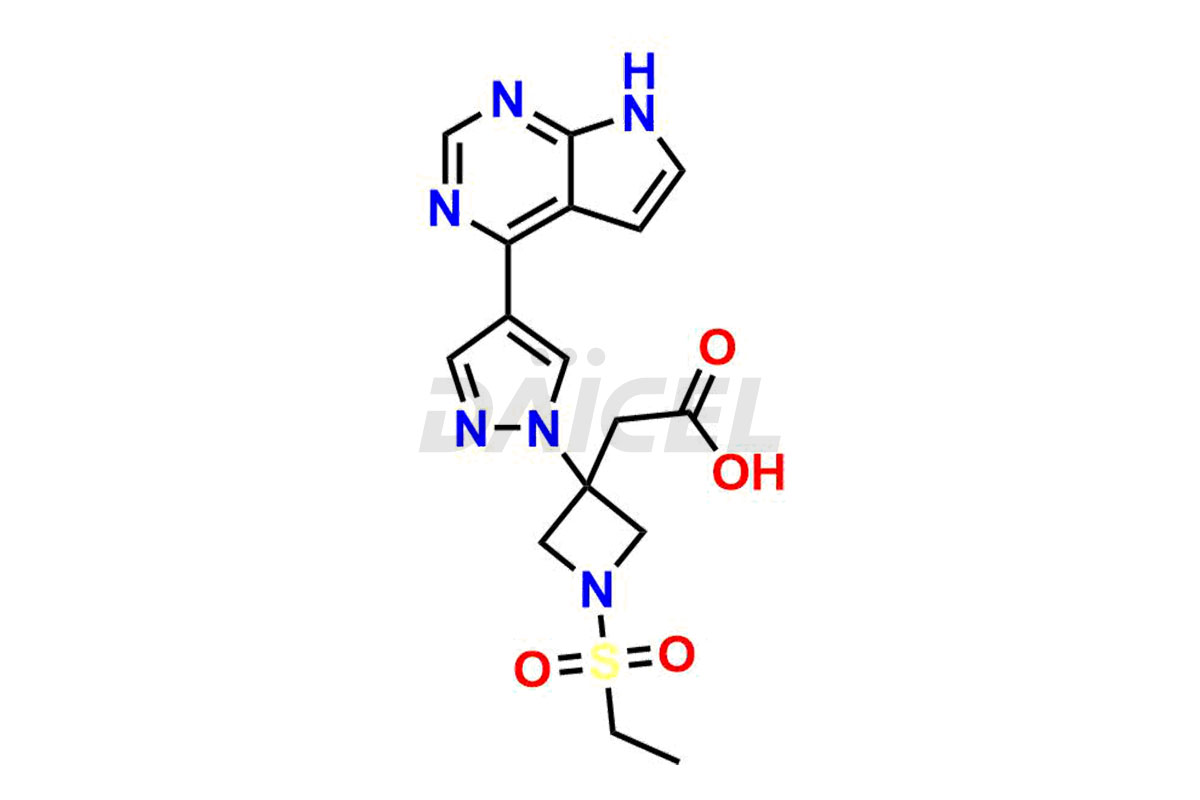
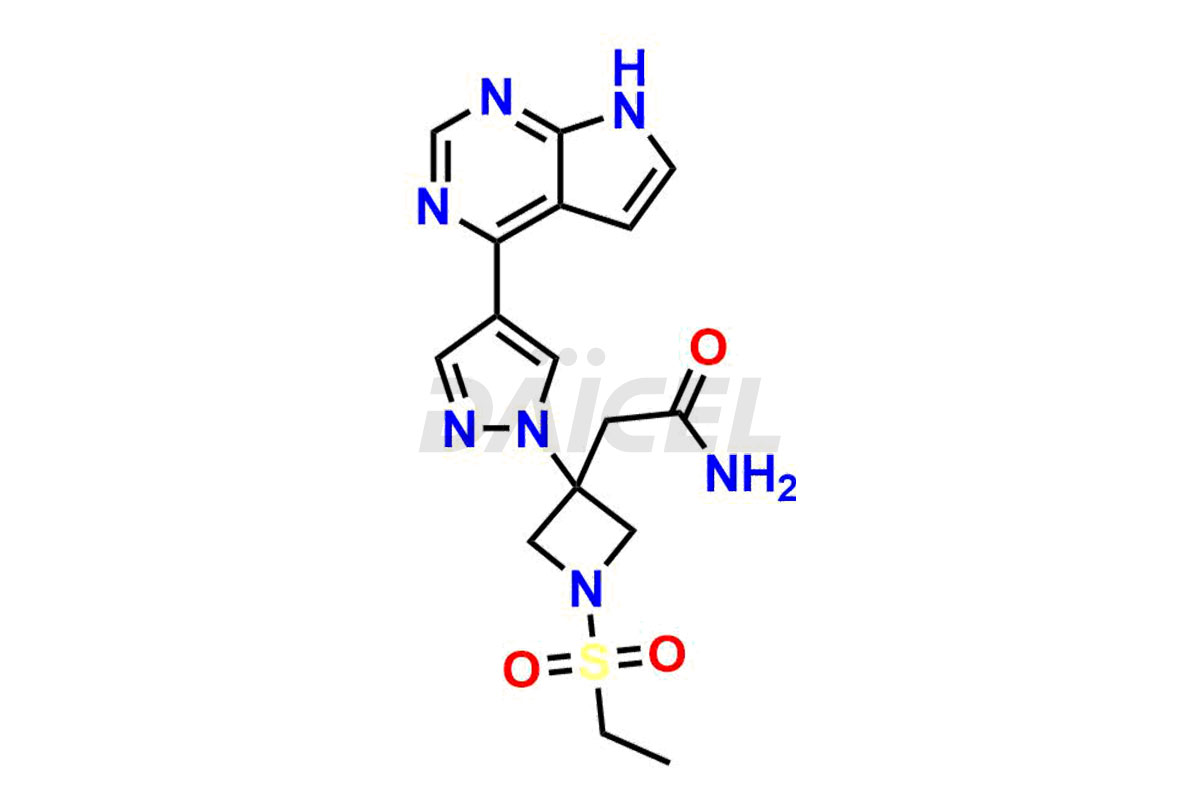
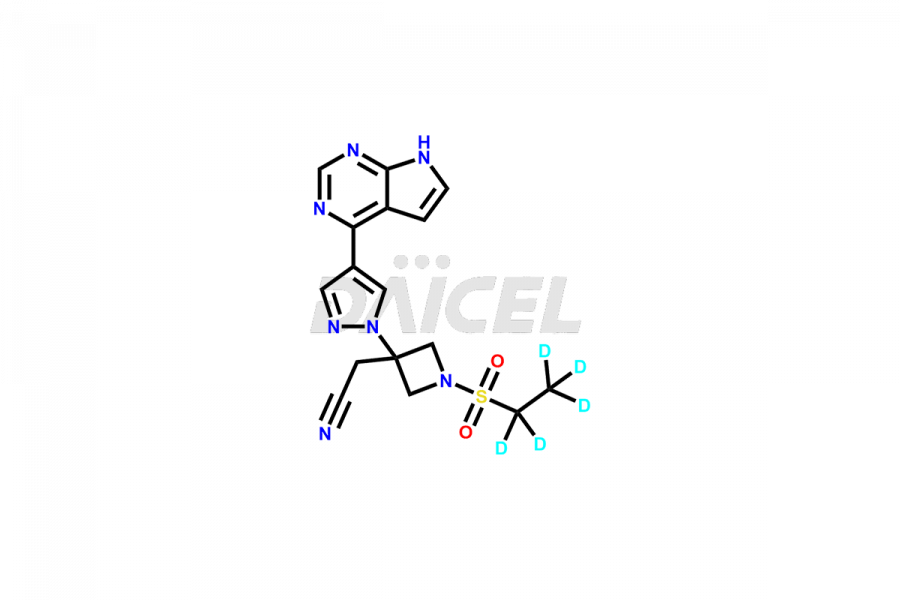
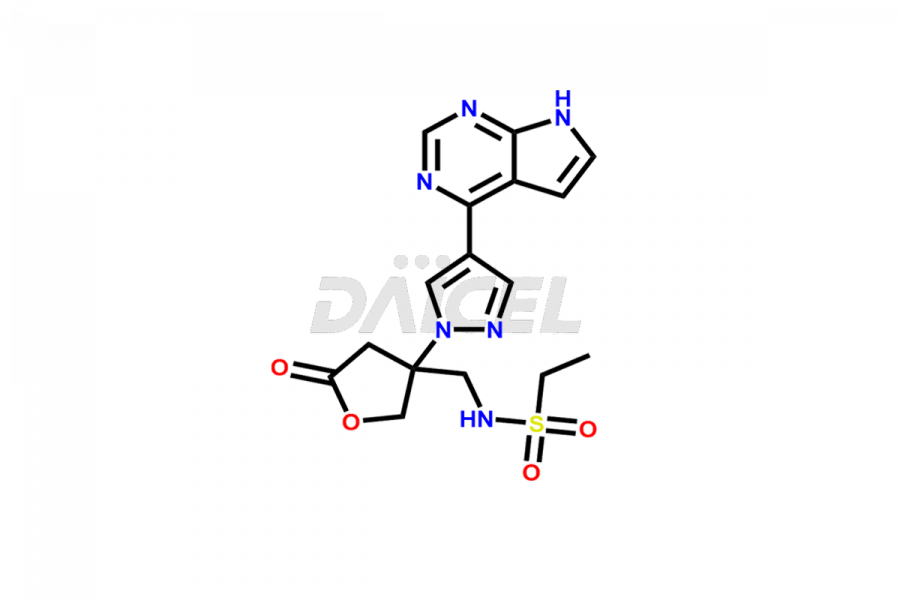
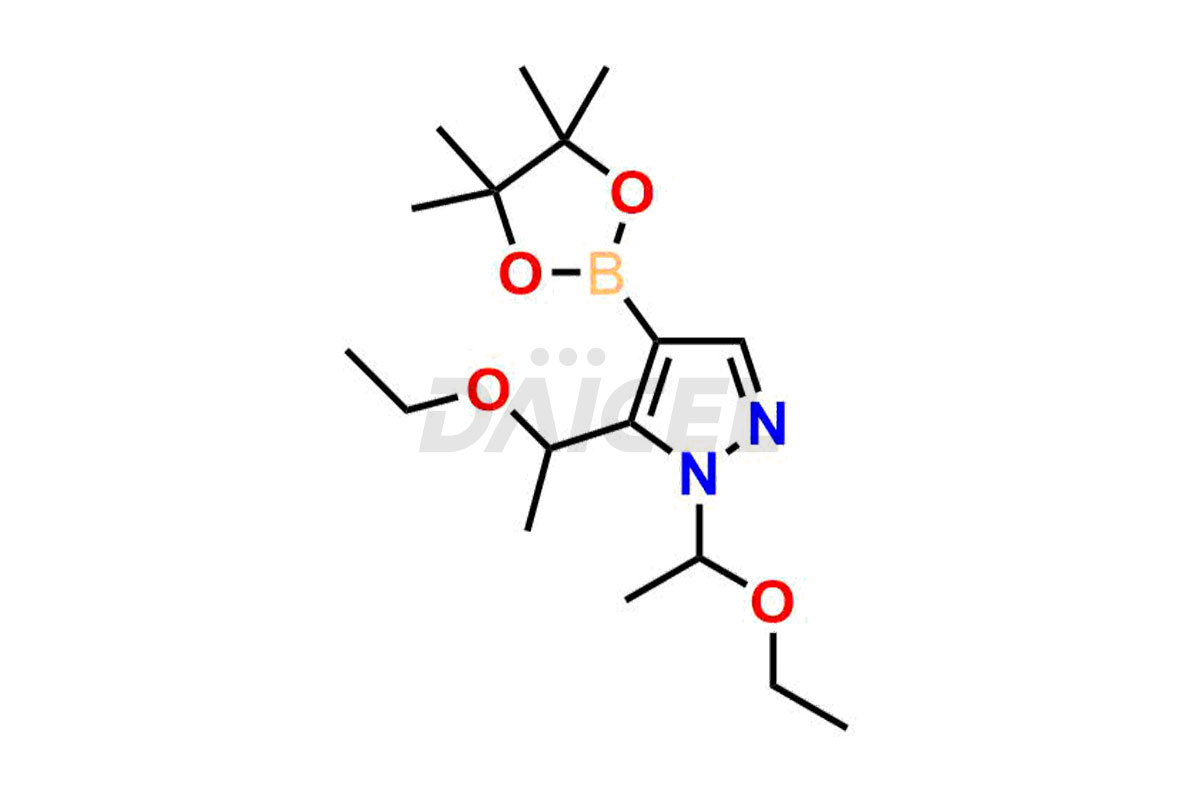
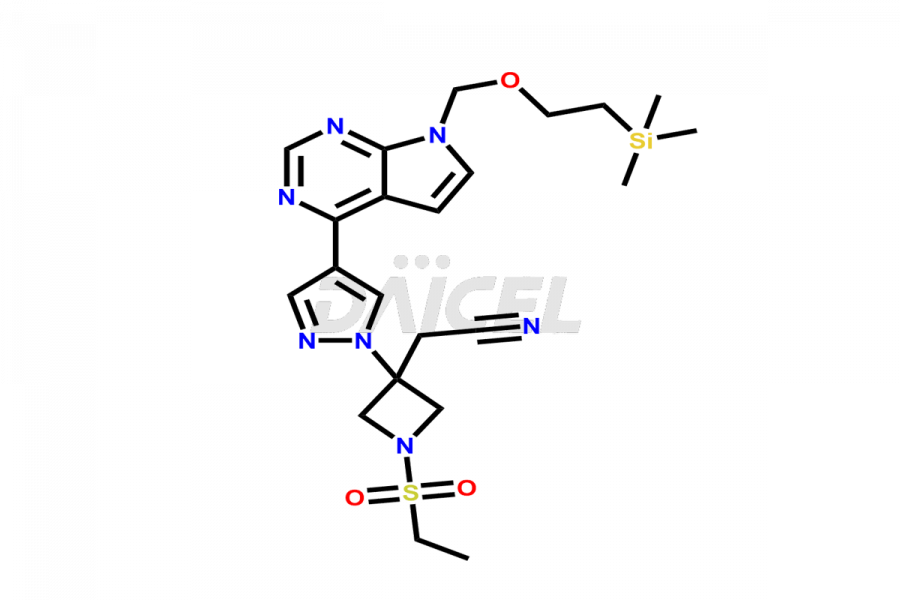
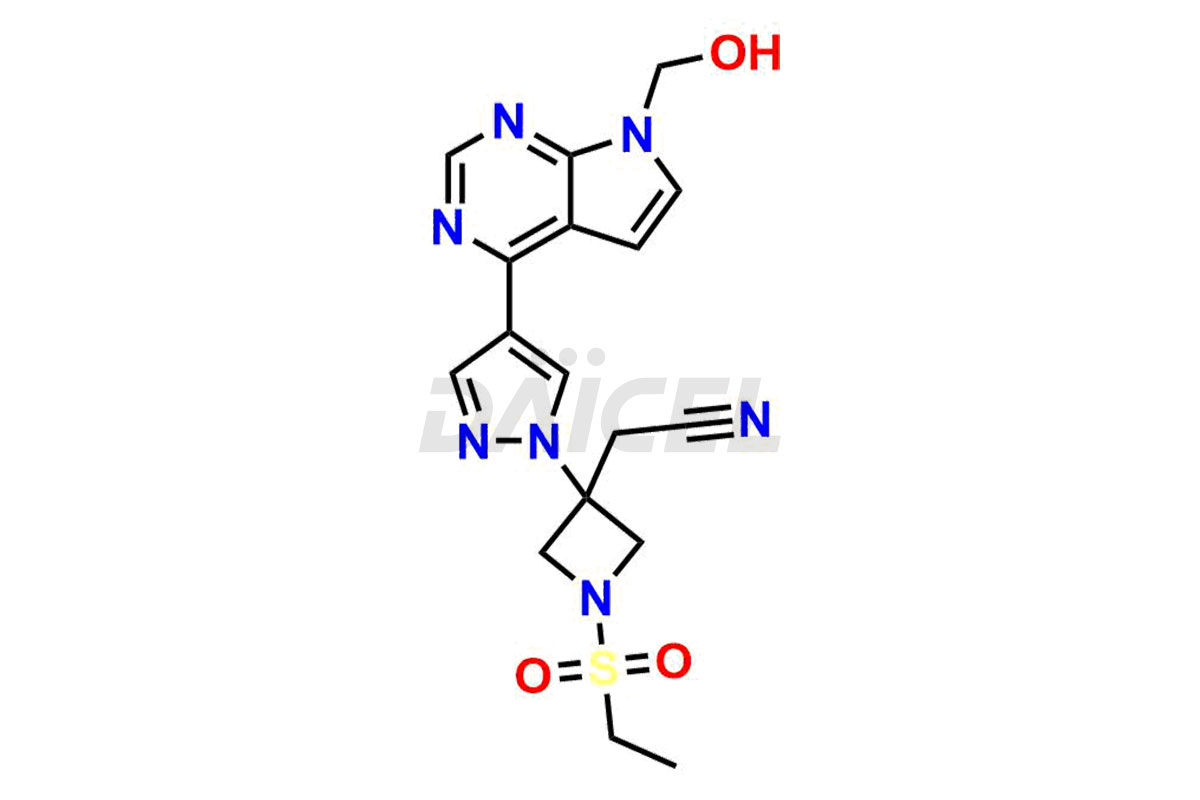
Daicel Pharma synthesizes high-quality Baricitinib impurities, including 2-(1-(ethylsulfonyl)-3-(4-(7-(hydroxymethyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile, Baricitinib Acid Impurity, Baricitinib Amide Impurity, N-((3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-5-oxotetrahydrofuran-3-yl)methyl) ethanesulfonamide, 2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl) acetonitrile, and so on. These impurities are essential for evaluating the quality, stability, and safety of Baricitinib, an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Baricitinib impurities for global delivery to meet the specific needs of our customers.
Baricitinib [CAS: 1187594-09-7] is a small-molecule Janus kinase (JAK) inhibitor. It treats moderate-to-severe rheumatoid arthritis, alopecia areata, and severe COVID-19 in hospitalized patients who require oxygen supplementation. Baricitinib reduces inflammation, modulates the immune system, and has antineoplastic properties.
Baricitinib: Use and Commercial Availability
Baricitinib is an antirheumatic drug approved by the US FDA for use in adults with moderately to severely active rheumatoid arthritis who have not responded adequately to other disease-modifying antirheumatic drugs, such as TNF antagonist therapies. Baricitinib is available under the trade name Olumiant.
Baricitinib Structure and Mechanism of Action 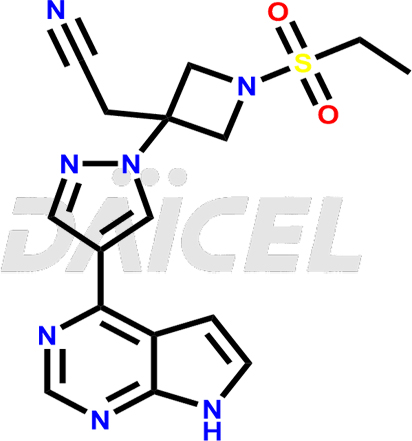
The chemical name of Baricitinib is 1-(Ethylsulfonyl)-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]-3-azetidine acetonitrile. Its chemical formula is C16H17N7O2S, and its molecular weight is approximately 371.4 g/mol.
Baricitinib inhibits Janus kinases, enzymes that transmit signals from cytokine or growth factor-receptor interactions on the cellular membranes.
Baricitinib Impurities and Synthesis
During the manufacturing1 of Baricitinib, impurities may form in the drug substance, which can affect its safety and efficacy. Some common Baricitinib impurities include related compounds, degradants, and residual solvents. Therefore, it is essential to control them and ensure the purity of the drug substance.
Daicel Pharma provides a Certificate of Analysis (CoA) for Baricitinib impurity standards, including 2-(1-(ethylsulfonyl)-3-(4-(7-(hydroxymethyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile, Baricitinib Acid Impurity, Baricitinib Amide Impurity, N-((3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-5-oxotetrahydrofuran-3-yl)methyl) ethanesulfonamide, 2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl) acetonitrile, and so on. The CoA is generated from a cGMP-compliant analytical facility and includes comprehensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We can also give additional characterization data like 13C-DEPT and CHN on request. Daicel Pharma is capable of creating unknown Baricitinib impurities or degradation products. Each delivery has a complete characterization report.
References
FAQ's
References
- Rodgers, James D.; Shepard, Stacey; Li, Yun-Long; Zhou, Jiacheng; Liu, Pingli; Meloni, David; Xia, Michael, Azetidine and cyclobutane derivatives as JAK inhibitors, Incyte Corporation, United States, US8158616B2, April 17, 2012
- Gandhi, Santosh V.; Kapoor, Barkha G., Development and validation of UV spectroscopic method for estimation of baricitinib, Journal of Drug Delivery and Therapeutics, Volume: 9, Issue: 4Suppl., Pages: 488-491, 2019
Frequently Asked Questions
How are Baricitinib impurities controlled during the manufacturing process of the drug?
Impurities in Baricitinib are controlled during manufacturing by implementing good manufacturing practices (GMP), such as using high-quality raw materials and employing appropriate purification techniques.
Can Baricitinib impurities affect the stability of the drug product?
Yes, impurities in Baricitinib can affect the stability of the drug product, leading to degradation and decreased efficacy.
Are there any specific methods to control impurities in Baricitinib?
Yes, specific methods to control impurities in Baricitinib depend on the type of impurity and its source. For example, controlling process-related impurities involves optimizing synthetic processes and using appropriate purification techniques.
What are the temperature conditions required to store Baricitinib impurities?
Baricitinib impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.


![4-(1H-pyrazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidine | Daicel Pharma Standards](https://dev.daicelpharmastandards.com/wp-content/uploads/2022/06/DCTI-C-1824-900x600.jpg)