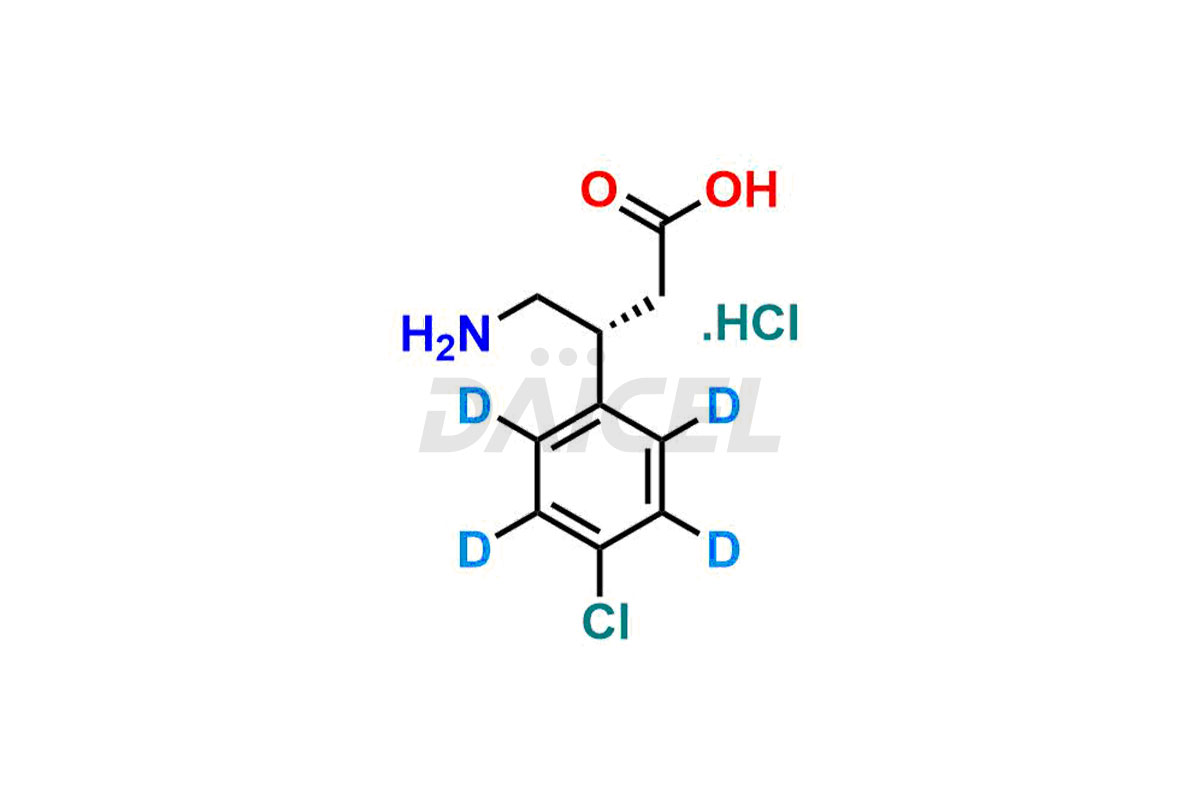
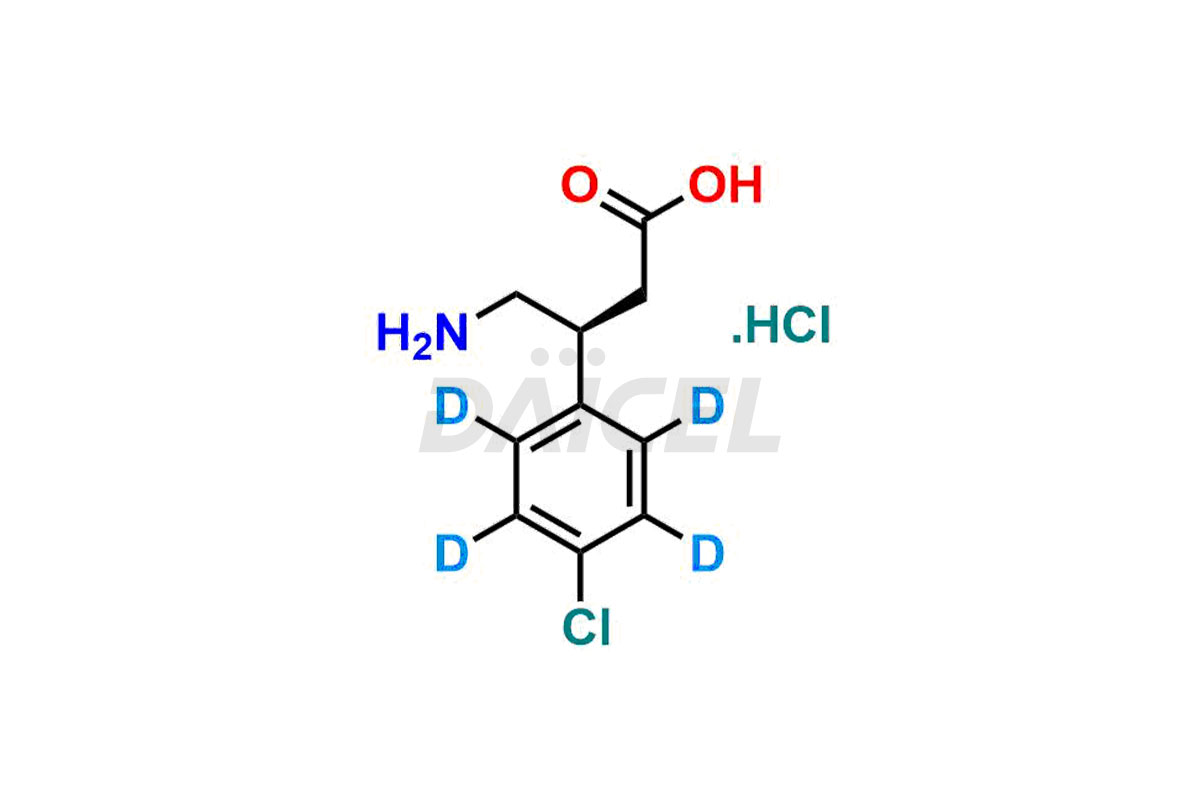
Baclofen
General Information
Baclofen Impurities and Baclofen
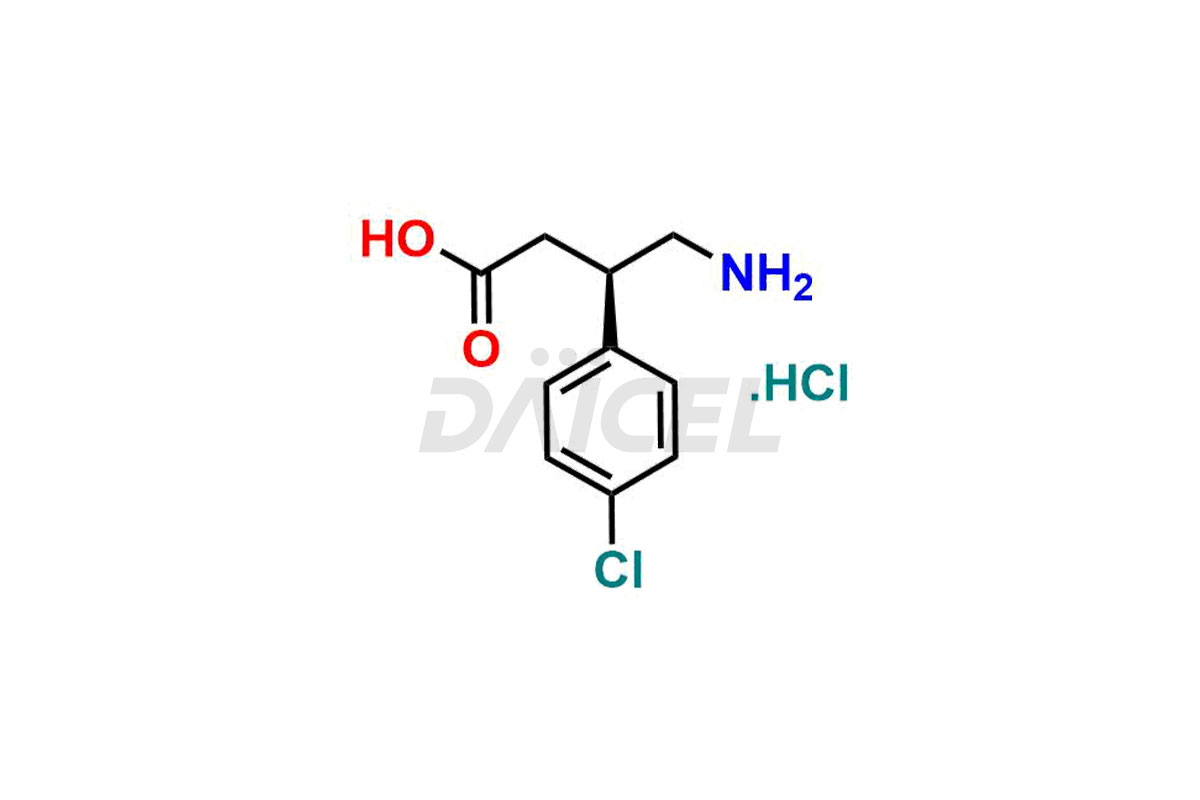
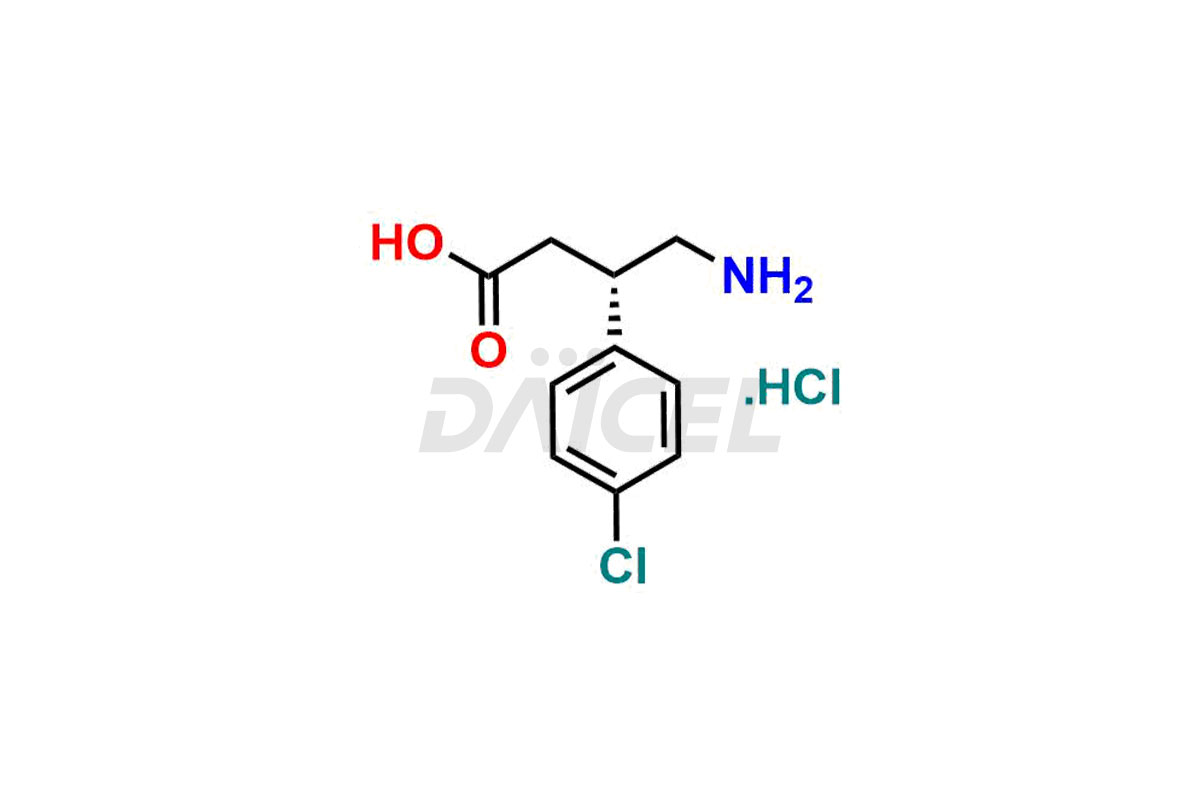
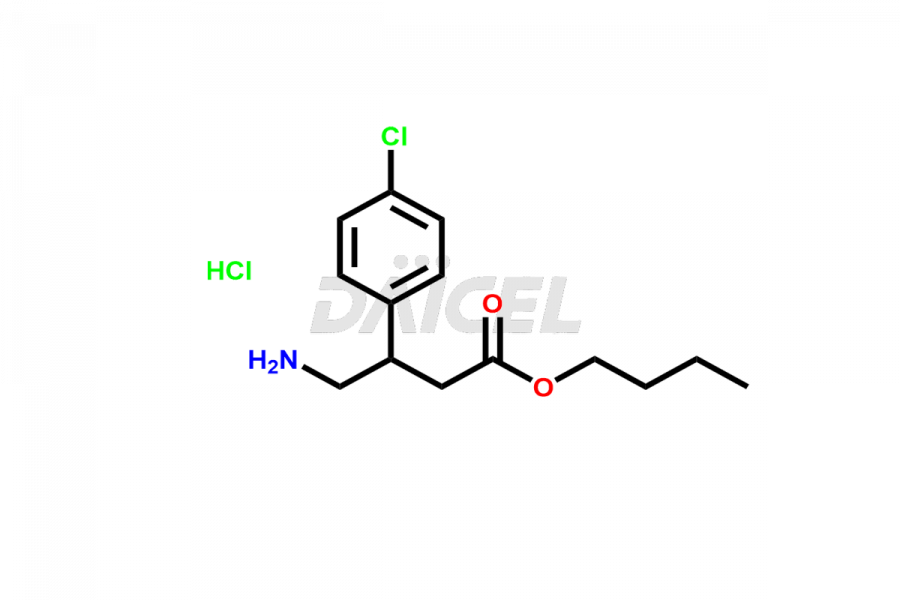
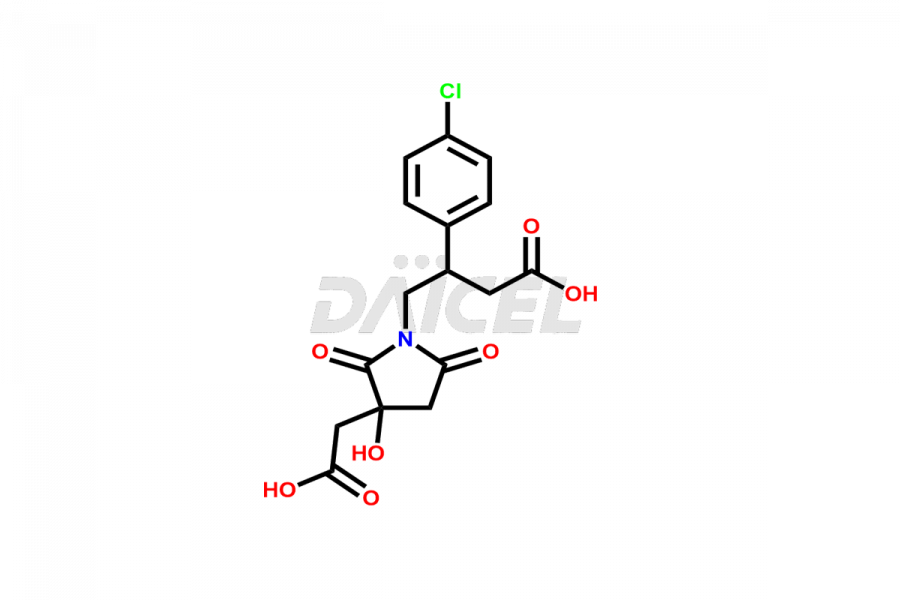
Daicel Pharma provides high-quality Baclofen impurities, including (R)-Baclofen Hydrochloride, (S)-Baclofen Hydrochloride, Baclofen butyl ester HCl, Baclofen impurity 4, and Diethyl 2-(4-chlorophenyl)-4-hydroxy-4-methyl-6-oxocyclohexane-1,3-dicarboxylate. These impurities are essential for evaluating the quality, stability, and safety of Baclofen, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Baclofen impurities for global delivery to meet the specific needs of our customers.
Baclofen [CAS: 1134-47-0] is a muscle relaxant primarily used to manage spasticity in individuals with multiple sclerosis. It is a synthetic chlorophenyl-butanoic acid derivative and functions by acting as a GABA agonist, specifically targeting GABA-B receptors.
Baclofen: Use and Commercial Availability
Baclofen impurities can form during manufacturing, storage, or transportation affecting the drug’s quality, safety, and efficacy. Some Baclofen impurities include related compounds, degradation products, and residual solvents. These impurities can be toxic, affect the drug’s stability, or alter its pharmacokinetics. So it is necessary to control and monitor their levels to ensure their quality and safety for patient use.
Baclofen Structure and Mechanism of Action 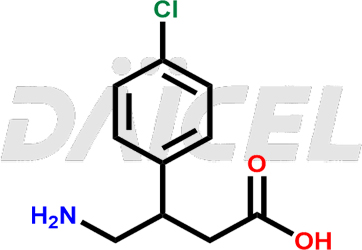
The name of Baclofen is β-(Aminomethyl)-4-chlorobenzenepropanoic acid. Its chemical formula is C10H12ClNO2, and its molecular weight is approximately 213.66 g/mol.
Baclofen inhibits monosynaptic and polysynaptic spinal reflexes. However, the exact mechanism of action is unknown.
Baclofen Impurities and Synthesis
Baclofen impurities can form during the manufacturing process, storage, or transportation, and they can affect the drug’s quality, safety, and efficacy. Some common impurities found in Baclofen include related compounds, degradation products, and residual solvents. These impurities can be toxic, affect the drug’s stability, or alter its pharmacokinetics. So it is necessary to control and monitor the levels of impurities in Baclofen to ensure its quality and safety for patient use.
Daicel Pharma offers a Certificate of Analysis (CoA) for Baclofen impurity standards, which includes (R)-Baclofen Hydrochloride, (S)-Baclofen Hydrochloride, Baclofen butyl ester HCl, Baclofen impurity 4, and Diethyl 2-(4-chlorophenyl)-4-hydroxy-4-methyl-6-oxocyclohexane-1,3-dicarboxylate. The CoA is produced from a cGMP-compliant analytical facility and includes comprehensive characterization data1, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization data, such as 13C-DEPT and CHN, can also be provided on request. Daicel Pharma can create unknown Baclofen impurities or degradation products and supply labeled compounds to evaluate the effectiveness of Baclofen. Further, Daicel Pharma offers R-Baclofen – D4 and S-Baclofen – D4, deuterium-labeled Baclofen compounds useful in bio-analytical research, such as BA/BE studies. Each delivery has a complete characterization report.
References
FAQ's
References
- Degen, P. H.; Riess, W., The determination of γ-amino-β-(p-chlorophenyl)butyric acid (baclofen) in biological material by gas-liquid chromatography, Journal of Chromatography, Volume: 117, Issue: 2, Pages: 399-405, 1976
- Harrison, P. M.; Tonkin, A. M.; McLean, A. J., Determination of 4-amino-3-(p-chlorophenyl)butyric acid (baclofen) in plasma by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 339, Issue: 2, Pages: 424-8, 1985
Frequently Asked Questions
Are all Baclofen impurities harmful to health?
Not all Baclofen impurities are harmful to health, but their presence can affect the quality and purity of the drug product, and some may pose a risk to patient health.
Can impurities in Baclofen be eliminated?
Complete elimination of impurities in Baclofen is not possible. However, a reduction in their levels to acceptable limits is through purification and quality control procedures.
How can the quality of Baclofen be ensured during storage and transportation?
The quality of Baclofen can be ensured during storage and transportation by following proper storage conditions such as temperature and humidity control and using appropriate packaging materials.
What are the temperature conditions required to store Baclofen impurities?
Baclofen impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.