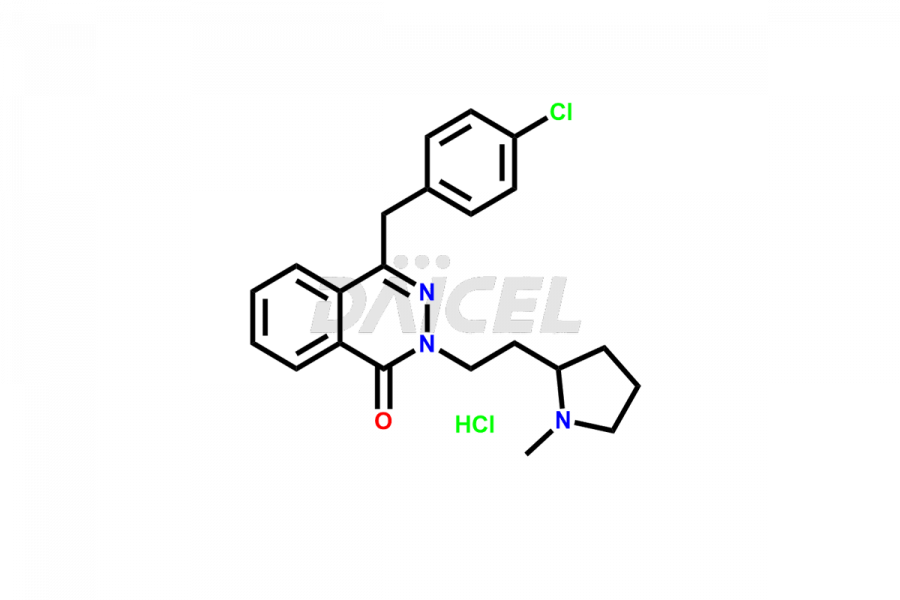
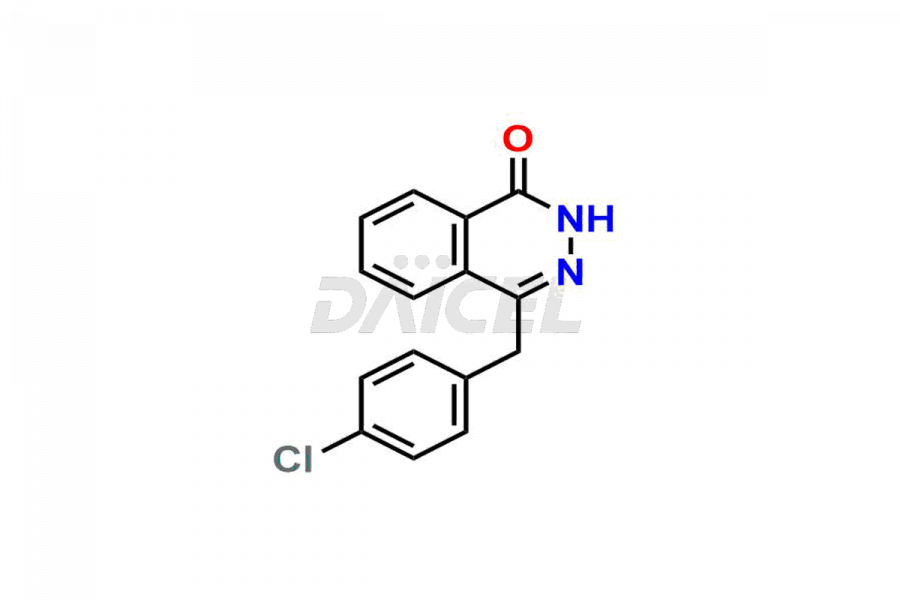
Azelastine
General Information
Azelastine Impurities and Azelastine
Daicel Pharma synthesizes high-quality Azelastine impurities, including Azelastine EP Impurity B, Azelastine HCl Isomer, and Azelastine related compound D. These impurities are essential for evaluating the quality, stability, and safety of Azelastine which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Azelastine impurities for global delivery to meet the specific needs of our customers.
Azelastine [CAS: 58581-89-8] is a medication used as a nasal spray to treat hay fever and as eye drops for allergic conjunctivitis. It is an antihistamine and a mast cell stabilizer. This drug is a member of the phthalazine group and contains a tertiary amino compound. Azelastine functions as an H1-receptor antagonist, an anti-allergic agent, an anti-asthmatic drug, a bronchodilator agent, and a platelet aggregation inhibitor.
Azelastine: Use and Commercial Availability
Azelastine has several indications for use depending on the formulation. Intranasal Azelastine treats seasonal allergic rhinitis and vasomotor rhinitis in patients. Ophthalmic Azelastine is used to treat itchy eyes associated with allergic conjunctivitis. Additionally, Azelastine is also available, in combination with fluticasone propionate, as a product called Dymista®, which is for patients over 12 years old for the symptomatic relief of seasonal allergic rhinitis. Azelastine is available under several brand names, Astelin, Astepro, Optivar, etc.
Azelastine Structure and Mechanism of Action 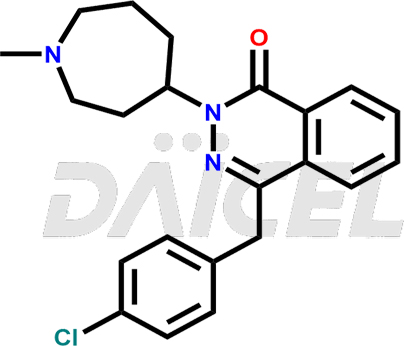
The chemical name of Azelastine is 4-[(4-Chlorophenyl)methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-1(2H)-phthalazinone. Its chemical formula is C22H24ClN3O, and its molecular weight is approximately 381.9 g/mol.
Azelastine inhibits the release of histamine and other mediators from cells in the allergic response.
Azelastine Impurities and Synthesis
During the manufacturing1 of Azelastine, impurities may form and can affect the quality and safety of the drug product. Azelastine Impurities may occur due to degradation, oxidation, or impure starting materials, and they can potentially cause adverse effects or decrease the drug’s effectiveness. Hence, controlling and monitoring the impurities formed during Azelastine production is crucial.
Daicel Pharma provides a Certificate of Analysis (CoA) for Azelastine impurity standards, including Azelastine EP Impurity B, Azelastine HCl Isomer, and Azelastine related compound D. The CoA is generated by a cGMP-compliant analytical facility and includes comprehensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We can also give additional characterization data like 13C-DEPT and CHN on request. Daicel Pharma is capable of creating unknown Azelastine impurities or degradation products. Each delivery has a complete characterization report.
References
FAQ's
References
- Engel, Juergen; Scheffler, Gerhard, 4-Benzyl-1-2(H)-Phthalazinone Derivatives, Asta-Werke A.-G., Federal Republic of Germany, EP222191B1, January 30, 1991
- Pivonka, J.; Segelman, F. H.; Hartman, C. A.; Segl, W. E.; Kucharczyk, N.; Sofia, R. D., Determination of azelastine and desmethylazelastine in human plasma by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 420, Issue: 1, Pages: 89-98, 1987
Frequently Asked Questions
Can Azelastine impurities affect the drug's bioavailability?
Impurities in Azelastine can affect the drug's bioavailability and its therapeutic effect.
How are the impurities in Azelastine controlled during manufacturing?
Impurities in Azelastine are controlled during manufacturing through various methods, such as monitoring and controlling the manufacturing process, testing at different stages of production, and using validated analytical methods.
What are the sources of Azelastine impurities?
Impurities in Azelastine can come from the starting materials, intermediates, degradation products, or residual solvents used in the manufacturing process.
What are the temperature conditions required to store Azelastine impurities?
Azelastine impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.