Atazanavir
General Information
Atazanavir Impurities and Atazanavir
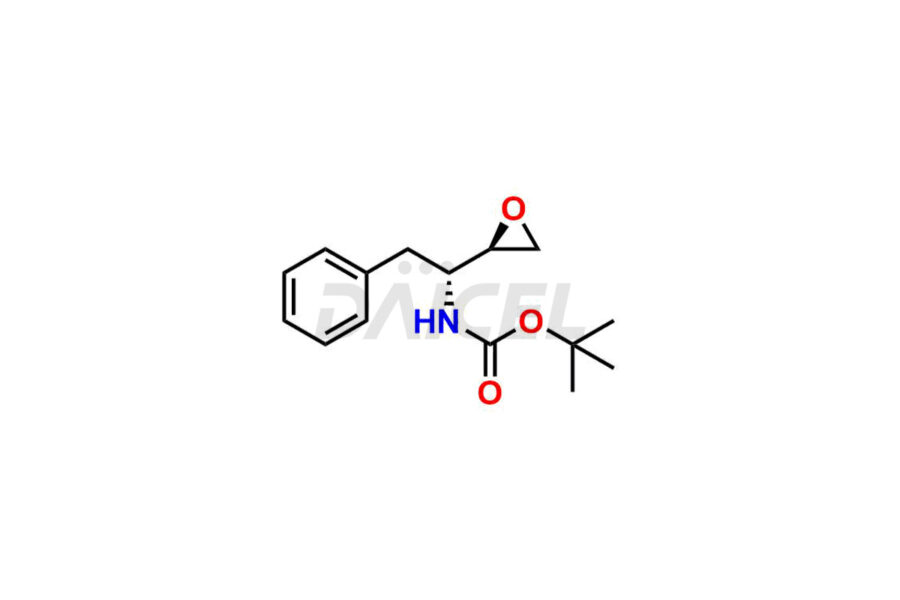
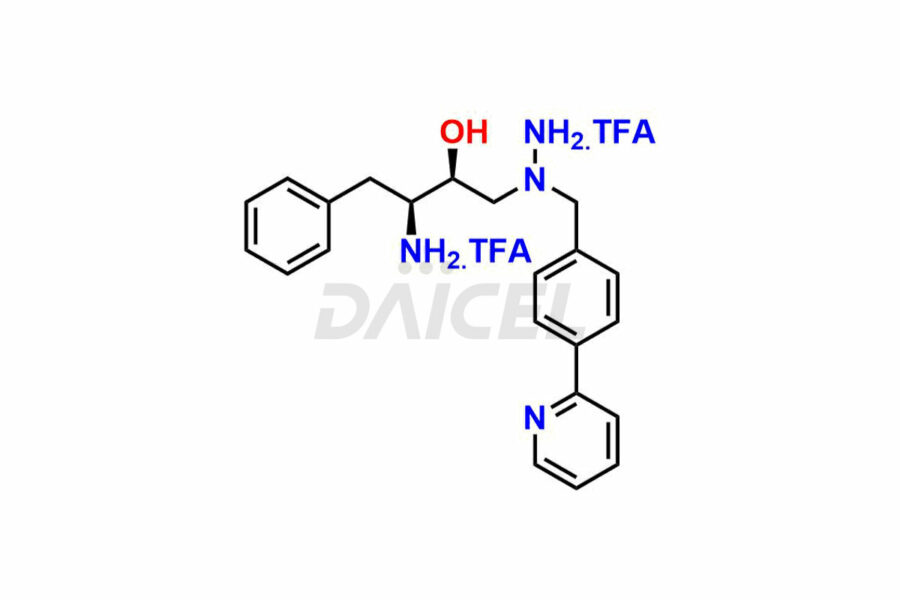
Daicel Pharma synthesizes high-quality Atazanavir impurities, including (+)-Tert-butyl ((R)-1-((R)-oxiran-2-yl)-2-phenylethyl) carbamate, Atazanavir Hydrazine Analog TFA Salt, and ATAZANAVIR SULFATE ATARC-1. These impurities are essential for evaluating the quality, stability, and safety of Atazanavir, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Atazanavir impurities for global delivery to meet the specific needs of our customers.
Atazanavir [CAS: 198904-31-3] is an antiretroviral drug that treats and prevents human immunodeficiency virus (HIV-1) infection and acquired immunodeficiency syndrome (AIDS). It does not elevate serum lipids like other protease inhibitors.
Atazanavir: Use and Commercial Availability
Atazanavir, available under the brand name, Reyataz, is approved by the US FDA to treat HIV infections in adults and children. It is available in two forms: capsules and oral powder.
Atazanavir Structure and Mechanism of Action 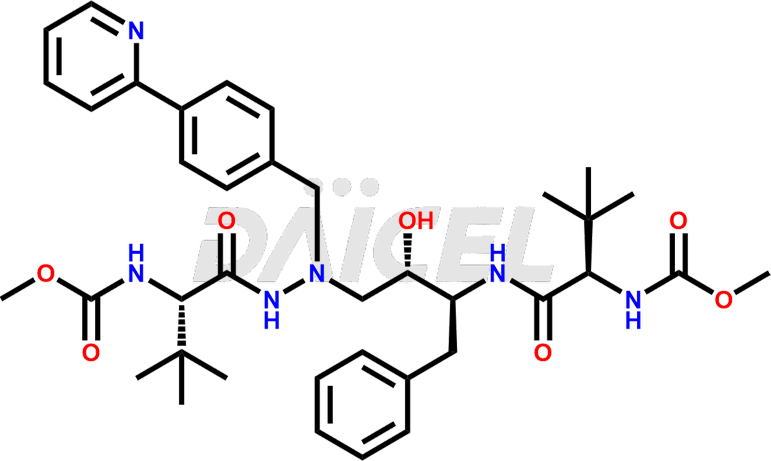
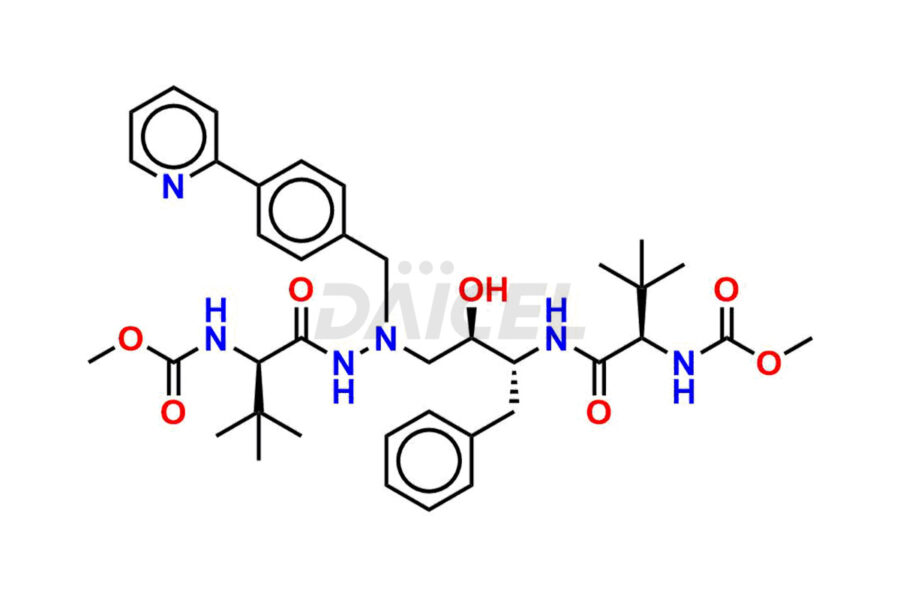
The chemical name of Atazanavir is methyl ((5S,8S,9S,14S)-8-benzyl-5-(tert-butyl)-9-hydroxy-15,15-dimethyl-3,6,13-trioxo-11-(4-(pyridin-2-yl)benzyl)-2-oxa-4,7,11,12-tetraazahexadecan-14-yl)carbamate. Its chemical formula is C38H52N6O7, and its molecular weight is approximately 704.9 g/mol.
Atazanavir inhibits the virus-specific processing of viral Gag and Gag-Pol polyproteins in HIV–1 infected cells and prevents mature virions formation.
Atazanavir Impurities and Synthesis
Like any other pharmaceutical compound, Atazanavir can contain impurities generated during its synthetic1 process. These impurities form due to various factors such as raw materials, synthetic procedures, storage, and degradation. Atazanavir impurities are of three types: organic, inorganic, and residual solvents. It is necessary to synthesize and analyze them and the residual solvents present in the drug substance to ensure the safety and efficacy of Atazanavir.
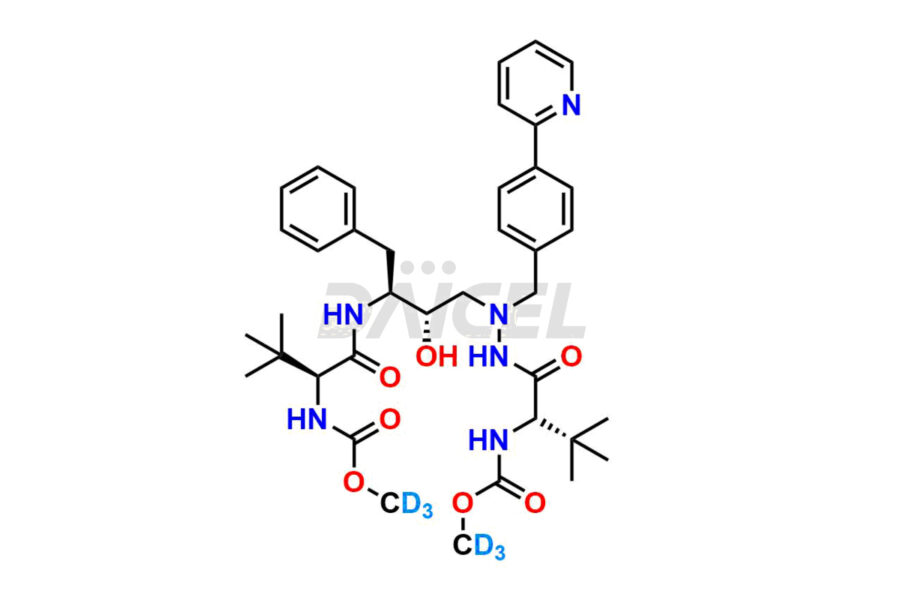
Daicel Pharma offers a Certificate of Analysis (CoA) for Atazanavir impurity standards, which includes (+)-Tert-butyl ((R)-1-((R)-oxiran-2-yl)-2-phenylethyl) carbamate, Atazanavir Hydrazine Analog TFA Salt, and ATAZANAVIR SULFATE ATARC-1. The CoA is produced from a cGMP-compliant analytical facility and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additional characterization data, such as 13C-DEPT and CHN, can also be provided on request. Daicel Pharma can prepare unknown Atazanavir impurities or degradation products and supply labeled compounds to evaluate the effectiveness of Atazanavir. Also, Daicel Pharma offers Atazanavir Labelled Standard, a deuterium-labeled Atazanavir-D6 standard useful in bio-analytical research, such as BA/BE studies. Each delivery has a complete characterization report.
References
FAQ's
References
- Fassler, Alexander; Bold, Guido; Capraro, Hans-Georg; Lang, Marc; Khanna, Satish Chandra, Antivirally active heterocyclic azahexane derivatives, Novartis A.-G., Switzerland, US5849911A, December 15,1998
- Schuster, A.; Burzawa, S.; Jemal, M.; Loizillon, E.; Couerbe, P.; Whigan, D., Quantitative determination of the HIV protease inhibitor atazanavir (BMS-232632) in human plasma by liquid chromatography-tandem mass spectrometry following automated solid-phase extraction, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 788, Issue: 2, Pages: 377-386, 2003
Frequently Asked Questions
What is the role of analytical testing in detecting Atazanavir impurities?
Analytical testing helps detect impurities in Atazanavir by providing accurate and reliable data on their presence and concentration.
Do impurities in Atazanavir affect its therapeutic efficacy?
Impurities in Atazanavir can affect its therapeutic efficacy by reducing the drug's potency or altering its mechanism of action.
How can Atazanavir impurities be controlled during the storage of the drug?
The impurities in Atazanavir are controlled during storage by ensuring appropriate temperature, humidity, and light conditions, using proper packaging and storage containers.
What are the temperature conditions required to store Atazanavir impurities?
Atazanavir impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.