Anastrozole
General Information
Anastrozole Impurities and Anastrozole
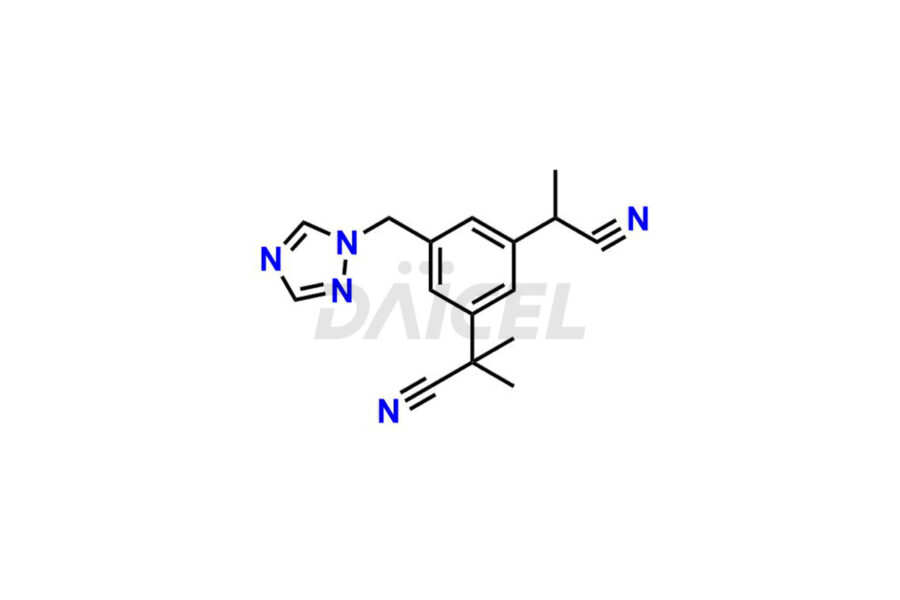
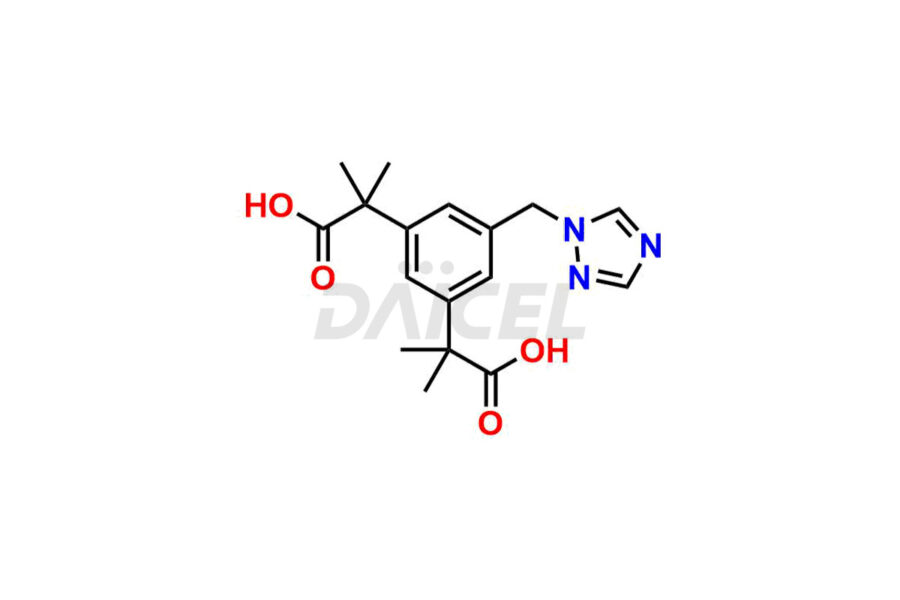
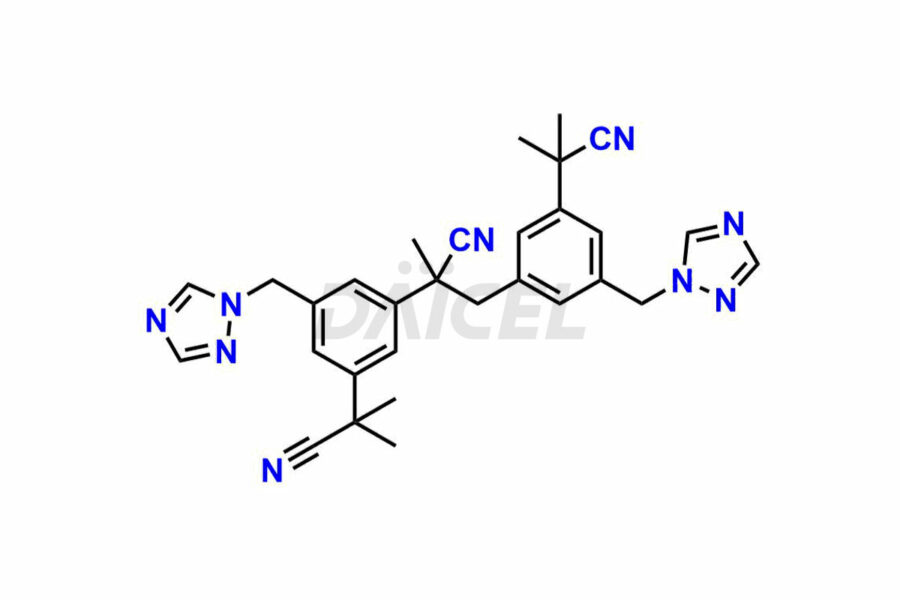
Daicel Pharma synthesizes high-quality Anastrozole impurities, including Alpha-Desmethyl Anastrozole, Anastrozole Diacid, and Anastrozole Dimer Impurity. These impurities are essential for evaluating the quality, stability, and safety of Anastrozole, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Anastrozole impurities for global delivery to meet the specific needs of our customers.
Anastrozole [CAS: 120511-73-1] is a non-steroidal aromatase inhibitor to treat postmenopausal women with estrogen receptor-positive breast cancer. It resembles the chemical structure of paclitaxel.
Anastrozole: Use and Commercial Availability
Anastrozole is a medication to treat breast cancer in postmenopausal women. Specifically, it is an adjunct therapy for hormone receptor-positive early breast cancer and as a first-line treatment for hormone receptor-positive or hormone receptor-unknown locally advanced or metastatic breast cancer. In addition, it treats advanced breast cancer in postmenopausal women who have not responded to treatment with tamoxifen. Anastrozole is a selective aromatase inhibitor. It is available under the brand name Arimidex.
Anastrozole Structure and Mechanism of Action 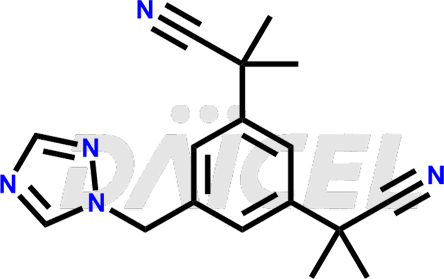
The chemical name of Anastrozole is α1α1α3α3-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile. Its chemical formula is C17H19N5, and its molecular weight is approximately 293.4 g/mol.
Anastrozole inhibits aromatase, which is an enzyme involved in the production of estrogen. By blocking this enzyme, Anastrozole can effectively reduce serum estradiol concentrations. It inhibits tumor growth and delays disease progression.
Anastrozole Impurities and Synthesis
During the synthesis1 of Anastrozole, impurities form that can affect the quality and safety of the final product. It is essential to synthesize and analyze these impurities to ensure the quality and safety of the medicine. The impurities form due to various reasons, such as degradation of the API, reaction with the solvent, or contaminants in the starting materials. Their identification and characterization are crucial to assess their potential toxicity and ensure that they are present within acceptable limits.
Daicel Pharma provides a Certificate of Analysis (CoA) for Anastrozole impurity standards, including Alpha-Desmethyl Anastrozole, Anastrozole Diacid, and Anastrozole Dimer Impurity. The CoA is generated from a cGMP-compliant analytical facility and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We can also give additional characterization data like 13C-DEPT and CHN on request. Daicel Pharma is capable of preparing unknown Anastrozole impurities or degradation products. Each delivery has a complete characterization report.
References
FAQ's
References
- Edwards, Philip Neil; Large, Michael Stewart, (Substituted aralkyl) heterocyclic compounds, Imperial Chemical Industries PLC, United Kingdom, EP296749B1, October 26, 1994
- Mendes, Gustavo D.; Hamamoto, Daniele; Ilha, Jaime; Pereira, Alberto dos Santos; De Nucci, Gilberto, Anastrozole quantification in human plasma by high-performance liquid chromatography coupled to photospray tandem mass spectrometry applied to pharmacokinetic studies, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 850, Issue: 1-2, Pages: 553-559, 2007
Frequently Asked Questions
How are the Anastrozole impurities synthesized?
Synthesis of Anastrozole impurities can be carried out by various methods such as chemical synthesis, isolation from related compounds, and degradation of the drug substance.
How are the impurities in Anastrozole controlled during the manufacturing process?
During manufacturing, control of Anastrozole impurities requires implementing appropriate measures such as process optimization, purification, and quality control testing.
How are the impurities in Anastrozole detected and quantified?
Anastrozole impurities are detected and quantified using analytical methods such as HPLC and LC-MS, which can identify and measure them based on their chemical properties.
What are the temperature conditions required to store Anastrozole impurities?
Anastrozole impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.