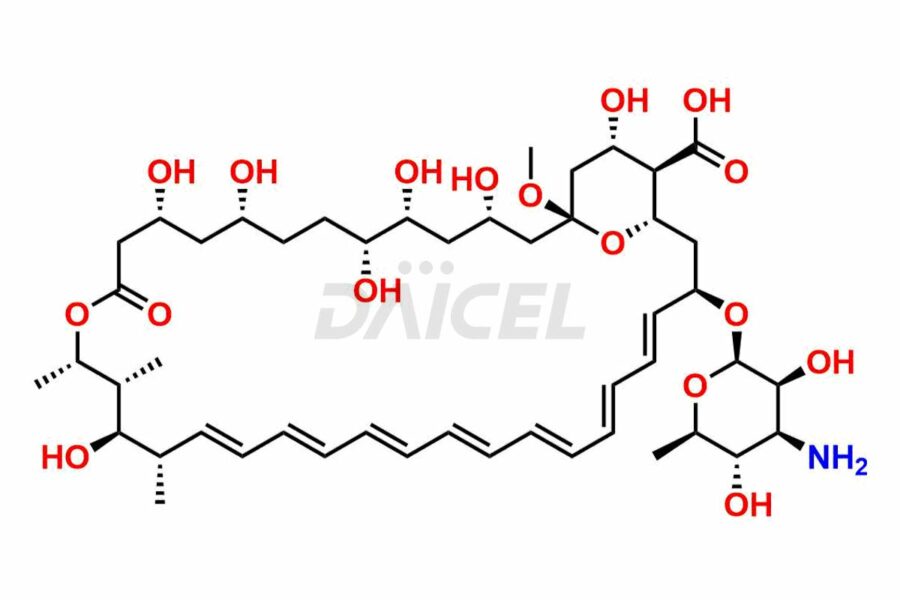
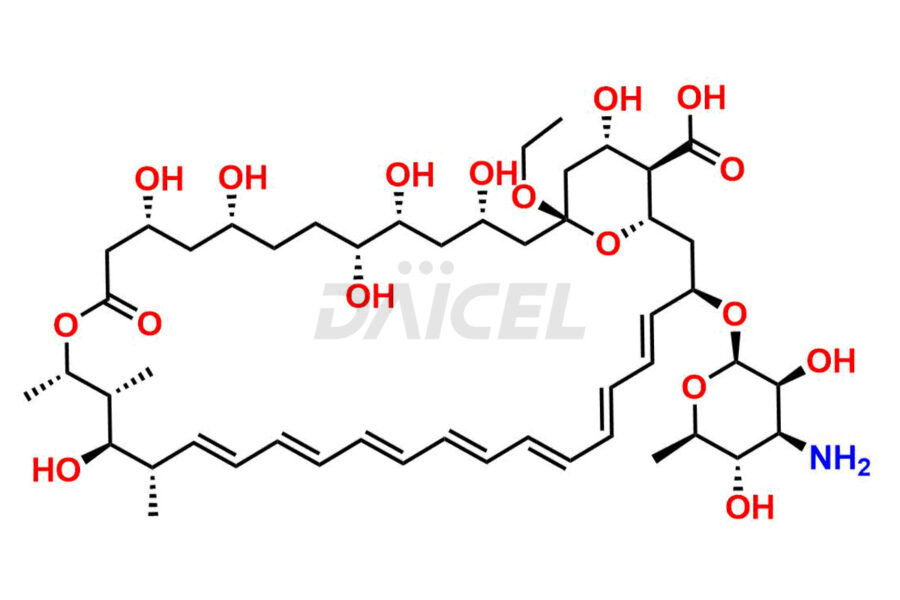
Amphotericin
General Information
Amphotericin Impurities and Amphotericin
Daicel Pharma synthesizes high-quality Amphotericin impurities, including Amphotericin B EP Impurity B and Amphotericin B EP Impurity C. These impurities are essential for evaluating the quality, stability, and safety of Amphotericin, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Amphotericin impurities with global delivery to meet the specific needs of our customers.
Amphotericin [CAS: 12633-72-6] or Amphotericin B [CAS: 1397-89-3] is an antifungal antibiotic from Streptomyces nodosus. It treats antifungal and Leishmaniasis infections.
Amphotericin: Use and Commercial Availability
Amphotericin B is an antifungal medication approved by the US FDA for treating several fungal infections, including histoplasmosis, cryptococcosis, and Leishmaniasis. Amphotericin B treats invasive fungal infections. The recommended daily dose depends on the type of infection, the organ involved, and the patient’s immune status. The medication is available under various brand names, including Abelcet, Ambisome, Amphotec, Fungizone, etc.
Amphotericin Structure and Mechanism of Action 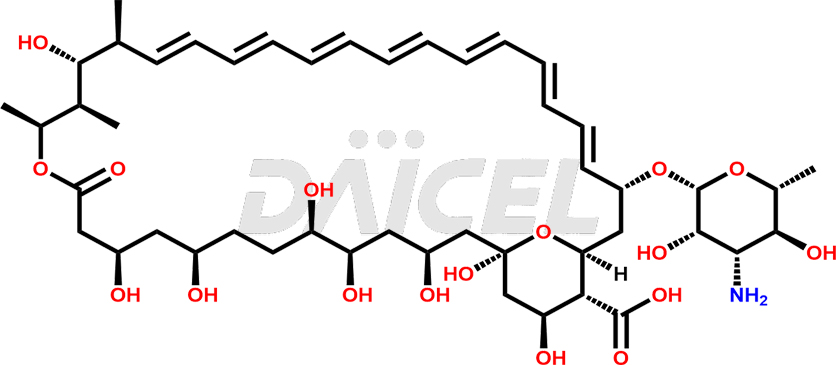
The chemical name of Amphotericin B is (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-Amino-3,6-dideoxy-β-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid. Its chemical formula is C47H73NO17, and its molecular weight is approximately 924.1 g/mol.
Amphotericin binds to ergosterol, which is essential for the fungal cell membrane. Its interaction causes changes in the cell membrane, leading to cell death.
Amphotericin Impurities and Synthesis
Amphotericin impurities form during manufacturing, storage, or by degradation under certain conditions. Some Amphotericin B impurities include related substances, degradation products, and residual solvents. Related substances form during the synthesis or isolation of Amphotericin B and can be structurally similar to the drug. Degradation products occur when Amphotericin B is exposed to light, heat, acidic, or alkaline conditions. These degradation products can be toxic and affect the efficacy of the drug. The residual solvents can also be present in Amphotericin B due to manufacturing. It is essential to control the impurities in Amphotericin as they can impact drug safety, efficacy, and quality.
Daicel Pharma provides a Certificate of Analysis (CoA) for Amphotericin impurity standards, including Amphotericin B EP Impurity B and Amphotericin B EP Impurity C. The CoA is from a cGMP-compliant analytical facility and includes comprehensive characterization data1 such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We can also give additional characterization data like 13C-DEPT and CHN on request. Daicel Pharma is capable of creating unknown Amphotericin impurities or degradation products. Each delivery comes with a complete characterization report.
References
FAQ's
References
- Gallelli, Joseph F., Assay and stability of amphotericin B in aqueous solutions, Drug Intelligence, Volume: 1, Issue: 3, Pages: 102-5, 1967
- Nilsson-Ehle, Ingrid; Yoshikawa, Thomas T.; Edwards, John E.; Schotz, Michael C.; Guze, Lucien B., Quantitation of amphotericin B with high-pressure liquid chromatography, Journal of Infectious Diseases, Volume: 135, Issue: 3, Pages: 414-22, 1977
Frequently Asked Questions
How is the purity of Amphotericin assessed?
The purity of Amphotericin is assessed using analytical techniques such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), and more.
What is the significance of validating analytical methods for impurity testing in Amphotericin?
Validation of analytical methods for impurity testing in Amphotericin is essential to ensure its accuracy, precision, specificity, and sensitivity.
Can Amphotericin impurities be removed through purification?
Purification methods such as chromatography, filtration, and crystallization help remove Amphotericin impurities.
What are the temperature conditions required to store Amphotericin impurities?
Amphotericin impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.