Amisulpride
General Information
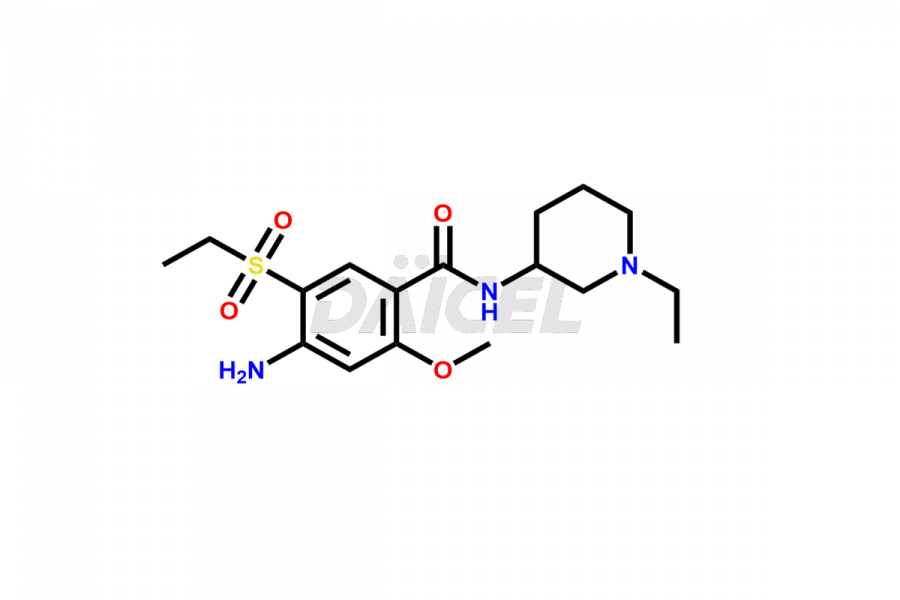
Amisulpride Impurities and Amisulpride
Daicel Pharma synthesizes high-quality Amisulpride impurities, including Amisulpride EP Impurity G, and Amisulpride EP Impurity H. These impurities are essential for evaluating the quality, stability, and safety of Amisulpride, which is an active pharmaceutical ingredient. Additionally, Daicel Pharma offers a customized synthesis of Amisulpride impurities with global delivery to meet the specific needs of our customers.
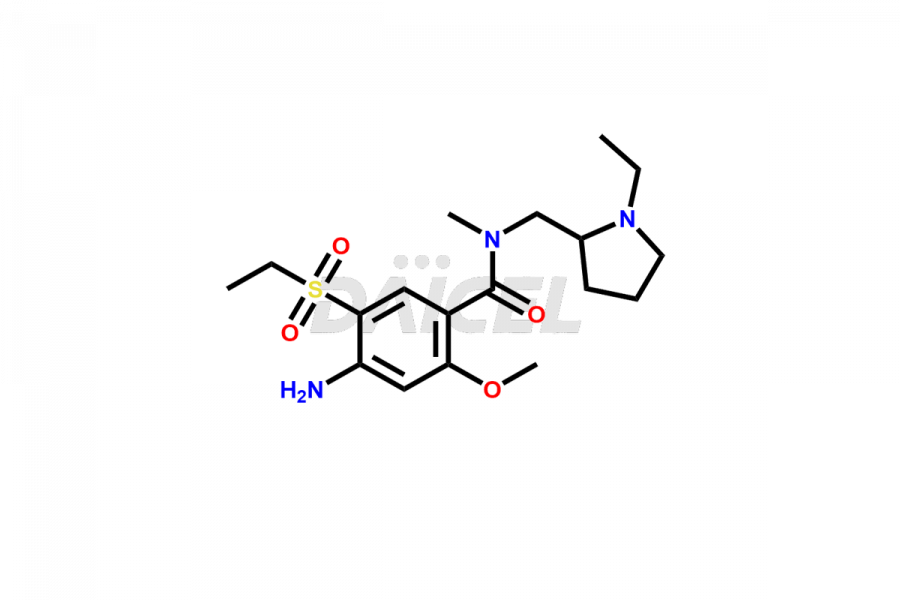
Amisulpride [CAS: 71675-85-9] is an antipsychotic medication that belongs to the benzamide derivatives. It acts as a selective antagonist of dopamine receptors D2 and D3. It effectively alleviates the positive and negative symptoms of schizophrenia. In addition to its antipsychotic properties, it has antidepressant effects in patients with major depression, dysthymia, and other psychiatric disorders.
Amisulpride: Use and Commercial Availability
Amisulpride has multiple indications depending on the route of administration. When given intravenously, it is used in adults to prevent and treat postoperative nausea and vomiting, either alone or in combination with a different antiemetic drug. When taken orally, Amisulpride treats acute and chronic schizophrenia, with symptoms such as delusions, hallucinations, and thought disorders, and negative symptoms such as emotional withdrawal. It can also help control secondary negative symptoms and affective disorders like depression. The brand name under which Amisulpride is available is Barhemsys.
Amisulpride Structure and Mechanism of Action 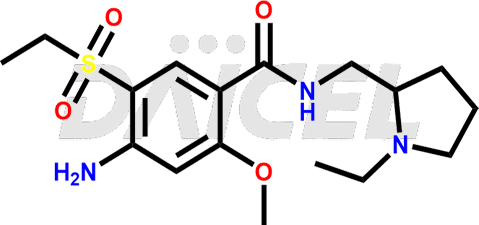
The chemical name of Amisulpride is 4-Amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide. Its chemical formula is C17H27N3O4S, and its molecular weight is approximately 369.5 g/mol.
Amisulpride inhibits dopaminergic hyperactivity. As a selective dopamine-2 (D2) and dopamine-3 (D3) receptor antagonist, Amisulpride prevents emesis.
Amisulpride Impurities and Synthesis
Impurities in Amisulpride may form during the manufacturing1, storage, or degradation of the drug product. They may include related substances, residual solvents, and degradation products that may affect drug’s efficacy, safety, and quality. Therefore, it is necessary to control the levels of impurities in Amisulpride to ensure that the drug product meets the required specifications and is safe for use. Amisulpride impurities are controlled by methods like process optimization, selecting appropriate starting materials, and using suitable analytical methods for impurity detection and quantification.
Daicel Pharma provides a Certificate of Analysis (CoA) for Amisulpride impurity standards, including Amisulpride EP Impurity G and Amisulpride EP Impurity H. The CoA is from a cGMP-compliant analytical facility and includes comprehensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We can also give additional characterization data like 13C-DEPT and CHN upon request. Daicel Pharma is capable of creating unknown Amisulpride impurities or degradation products. Each delivery comes with a complete characterization report.
References
FAQ's
References
- Thominet, M.; Acher, J.; Monier, J. C., Substituted Orthoanisamides Methods Of Preparing Them Compositions Containing Them And Their Application As Psychotropic Agents, Societe d'Etudes Scientifiques et Industrielles de l'Ile-de-France, France, GB2012765B, September 8, 1982
- Bohbot, M.; Doare, L.; Diquet, B., Determination of a new benzamide, amisulpride, in human plasma by reversed-phase ion-pair high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 416, Issue: 2, Pages: 414-19, 1987
Frequently Asked Questions
What are the common Amisulpride impurities found in the drug?
The common impurities found in Amisulpride include related substances such as isomers, degradants, residual solvents, and heavy metals.
How are impurities in Amisulpride controlled?
Impurities in Amisulpride are controlled through various methods, such as optimizing the synthesis process, using high-quality starting materials, and implementing strict quality control measures.
Which solvent helps in the analysis of Amisulpride impurities?
Acetonitrile is a solvent used in analyzing many impurities in Amisulpride.
What are the temperature conditions required to store Amisulpride impurities?
Amisulpride impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.