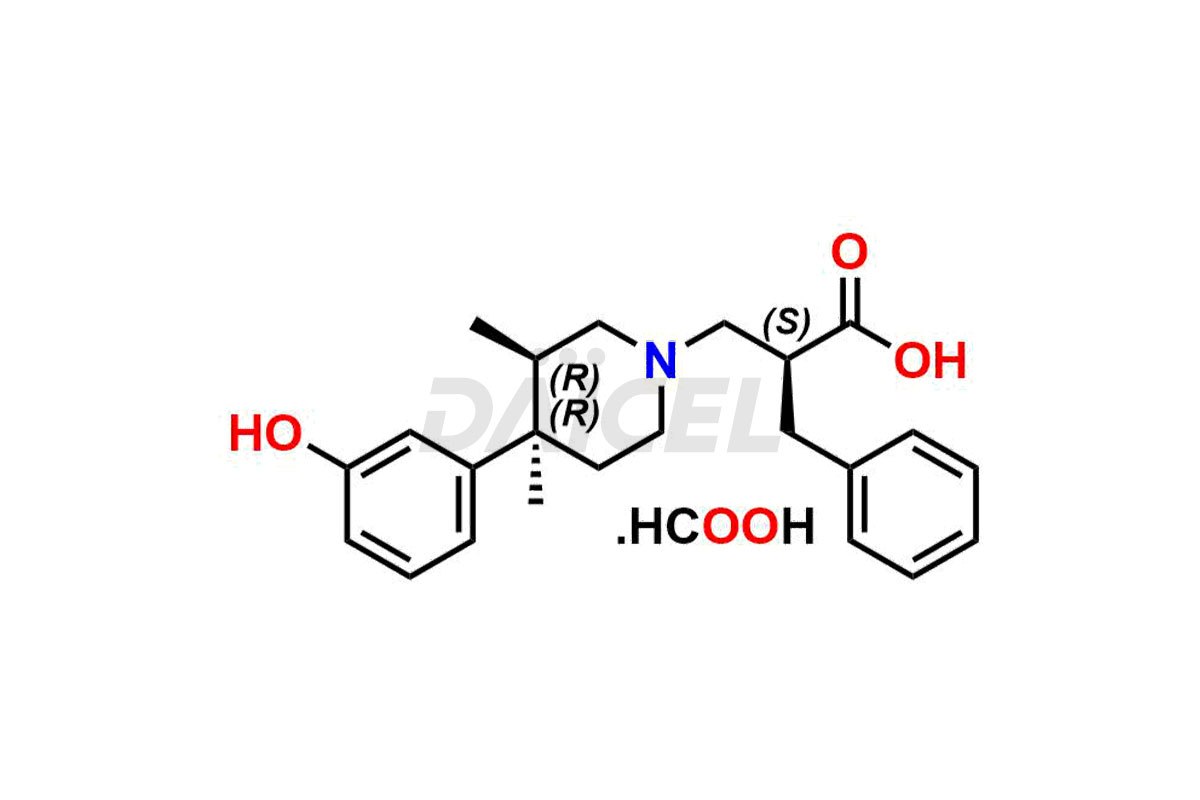
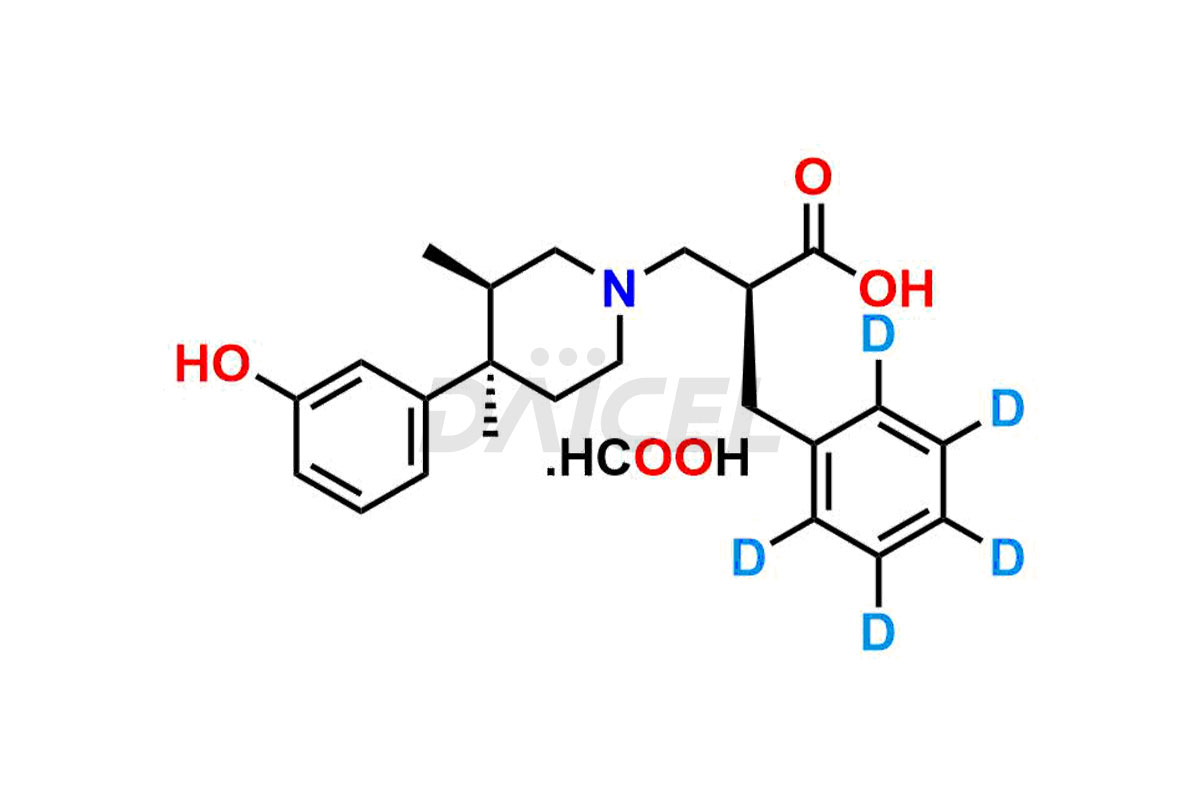
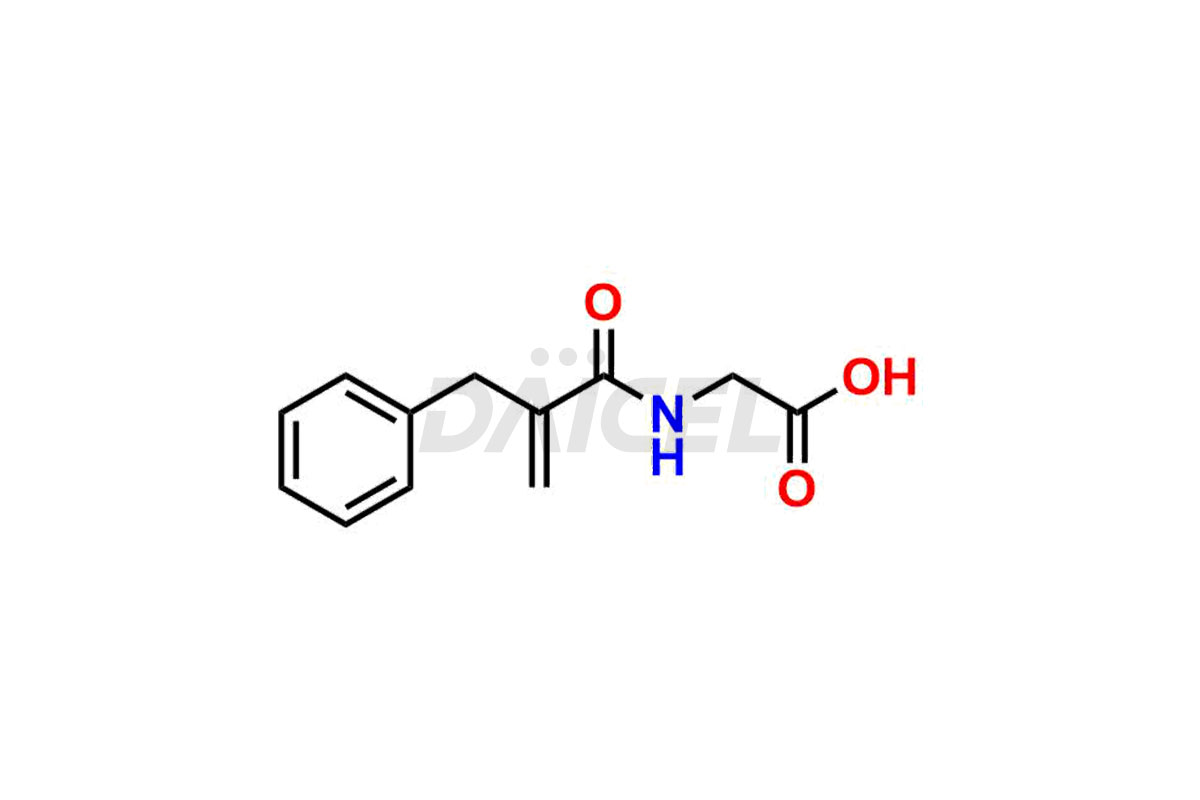
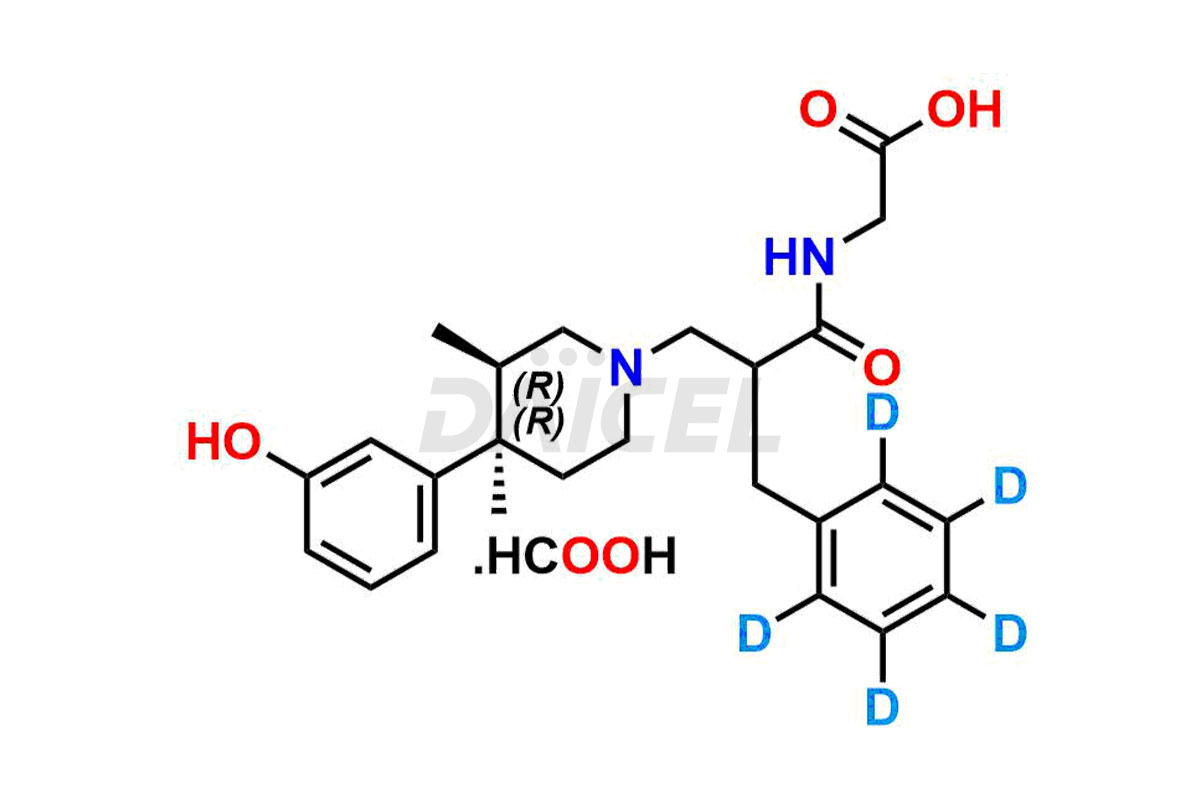
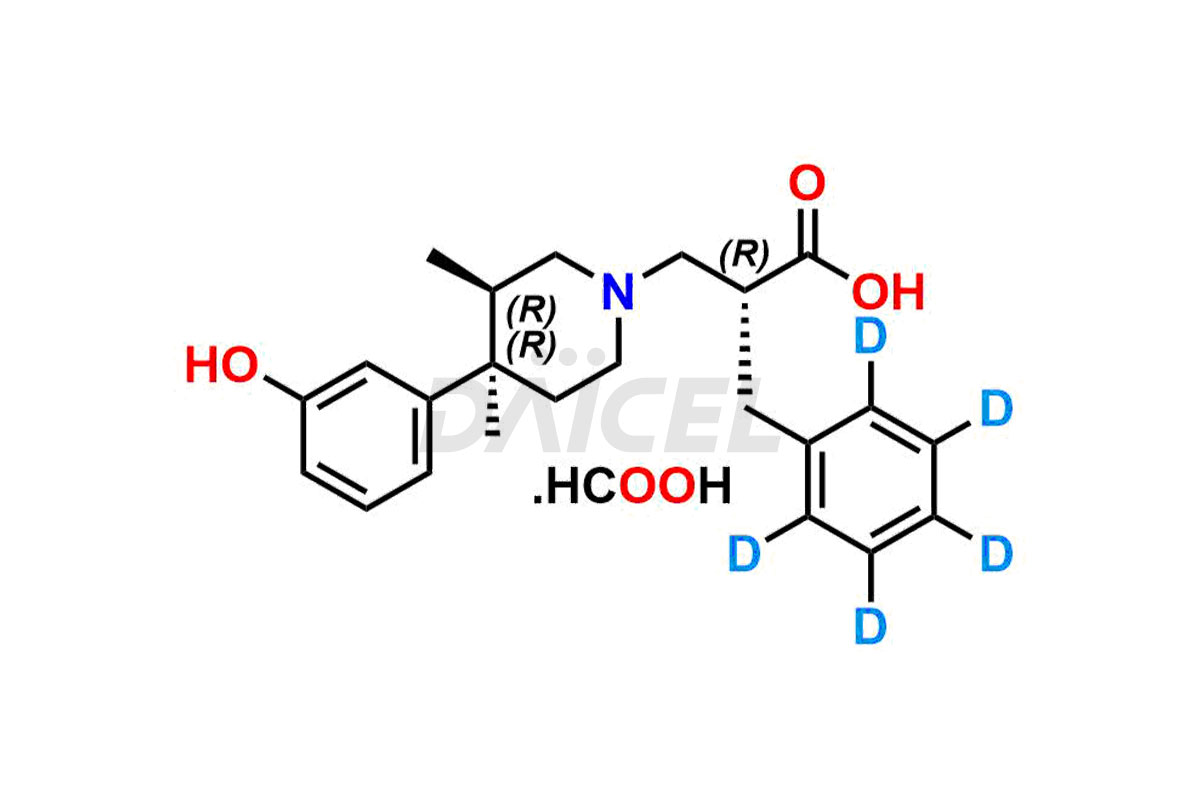
Alvimopan
General Information
Alvimopan Impurities and Alvimopan
Daicel Pharma is a reliable source for synthesizing high-quality Alvimopan impurities, specifically Alvimopan Metabolite Formic acid salt, Alvimopan olefin impurity, etc. These impurities help assess the quality, stability, and safety of Alvimopan. Daicel Pharma also offers a custom synthesis of Alvimopan impurities, which can be shipped worldwide to meet customers’ unique requirements.
Alvimopan [CAS: 156053-89-3] is a peripherally acting μ opioid antagonist. Its primary use is to prevent postoperative ileus, or the inability of the intestines to move food and stool, after small or large bowel resection surgery. By acting on the peripheral nervous system, Alvimopan can help accelerate the recovery period of the gastrointestinal system.
Alvimopan: Use and Commercial Availability
Alvimopan, available under the name Entereg, is a drug to speed up the time to upper and lower gastrointestinal recovery after partial large or small bowel resection surgery with primary anastomosis. Alvimopan reverses postoperative ileus after colectomy.
Alvimopan Structure and Mechanism of Action 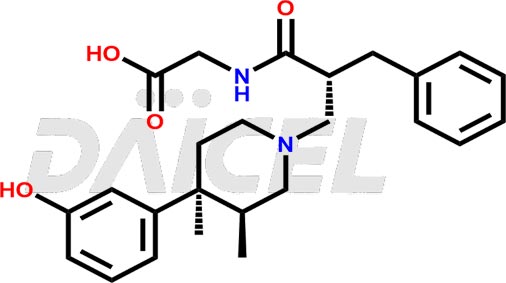
The chemical name of Alvimopan is N-[(2S)-2-[[(3R,4R)-4-(3-Hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-1-oxo-3-phenylpropyl]glycine. Its chemical formula is C25H32N2O4, and its molecular weight is approximately 424.5 g/mol.
Alvimopan is a peripherally acting μ-opioid antagonist, binding competitively to μ-opioid receptors in the gastrointestinal tract to minimize the undesirable side effects of opioid agonists, such as constipation, without affecting analgesia or precipitating withdrawal.
Alvimopan Impurities and Synthesis
During the manufacturing of Alvimopan, impurities form from starting materials, intermediates, or reagents used in synthesis1. They are beyond acceptable limits and can affect the drug’s quality, stability, and safety. Therefore, controlling them is necessary to ensure the drug’s quality and safety.
Daicel Pharma provides a Certificate of Analysis (CoA) for Alvimopan impurity standards, which includes Alvimopan Metabolite Formic acid salt, Alvimopan olefin impurity, etc. The CoA is from an analytical facility that adheres to current Good Manufacturing Practices (cGMP) and includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can also generate unknown Alvimopan impurities or degradation products and provide labeled compounds to assess the effectiveness of Alvimopan. Additionally, Daicel Pharma offers Alvimopan Metabolite-D5 Formic acid salt, Alvimopan-D5 Formic acid salt, and R-Alvimopan Metabolite-D5 Formic Acid salt, deuterium-labeled Alvimopan compounds used in bio-analytical research, such as BA/BE studies. A complete characterization report is part of each delivery.
References
FAQ's
References
- Frank, Scott Alan; Prather, Douglas Edward; Ward, Jeffrey Alan; Werner, John Arnold, Preparation of 3,4,4-trisubstitutedpiperidinyl-n-alkylcarboxylates and intermediates, useful as opioid antagonists, Eli Lilly and Co., United States, EP657428B1, April 4, 2001
- You, Jun; Cai, Sheng; Wu, Shuping, Separation of alvimopan and its enantiomers by HPLC using β-cyclodextrin as chiral mobile phase additive, Zhongguo Xiandai Yingyong Yaoxue, Volume: 31, Issue: 9, Pages: 1101-1104, 2014
Frequently Asked Questions
Why is it essential to analyze Alvimopan impurities?
Analyzing impurities in Alvimopan is critical to ensure drug safety and effectiveness. The impurities can affect drug quality, stability, and safety reducing its efficacy or causing harm to patients. Analyzing impurities can help identify their sources, enabling manufacturers to control their formation.
What steps can manufacturers take to control impurity levels in Alvimopan?
Manufacturers can take various steps to control impurity levels in Alvimopan, including using high-quality starting materials, optimizing reaction conditions, implementing effective purification techniques, and monitoring impurity levels throughout the manufacturing process.
Which solvent helps in the analysis of Alvimopan impurities?
Methanol is a solvent used in analyzing many impurities in Alvimopan.
What are the temperature conditions required to store Alvimopan impurities?
Alvimopan impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.