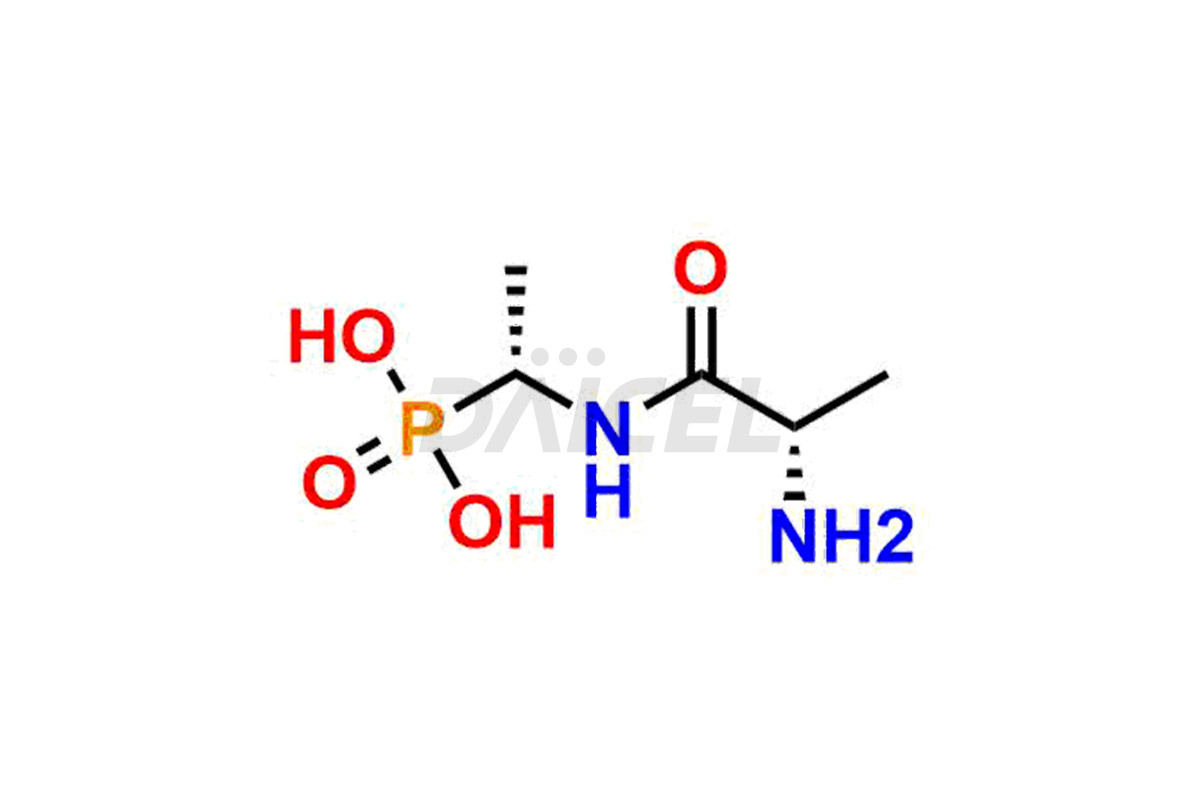
Alafosfalin
General Information
Alafosfalin Impurities and Alafosfalin
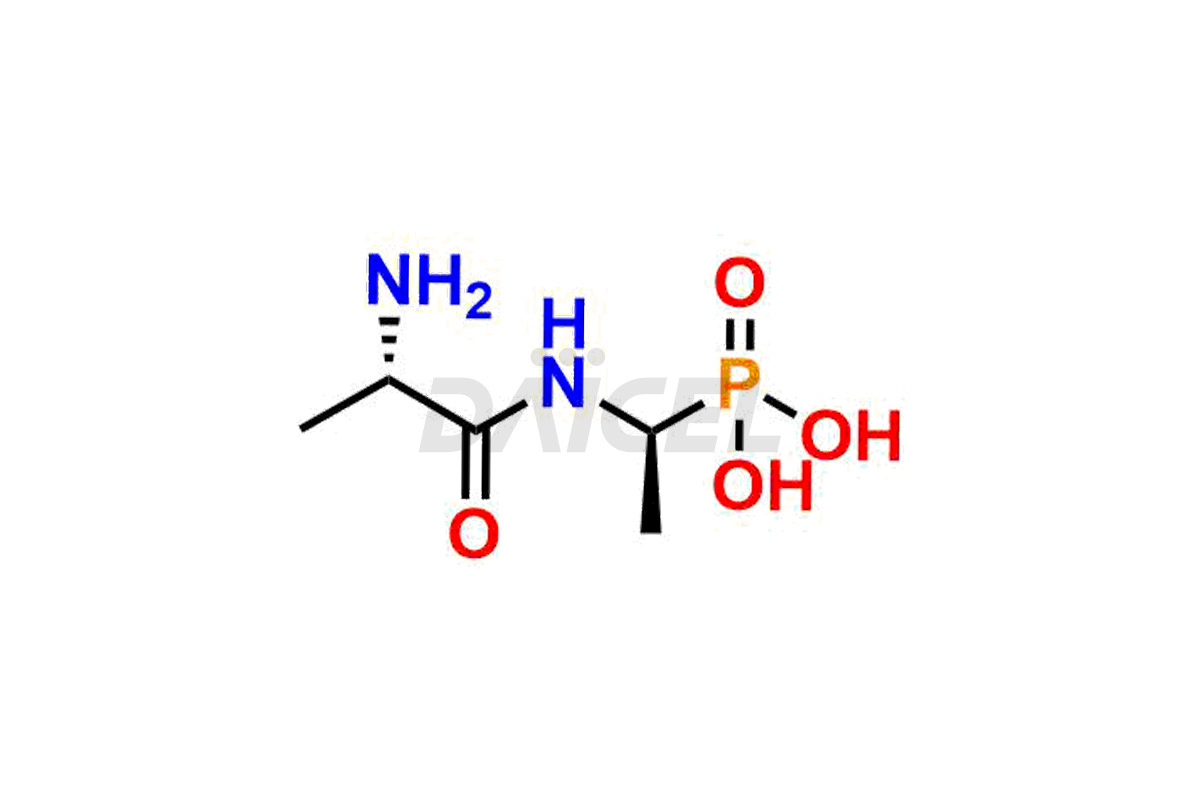
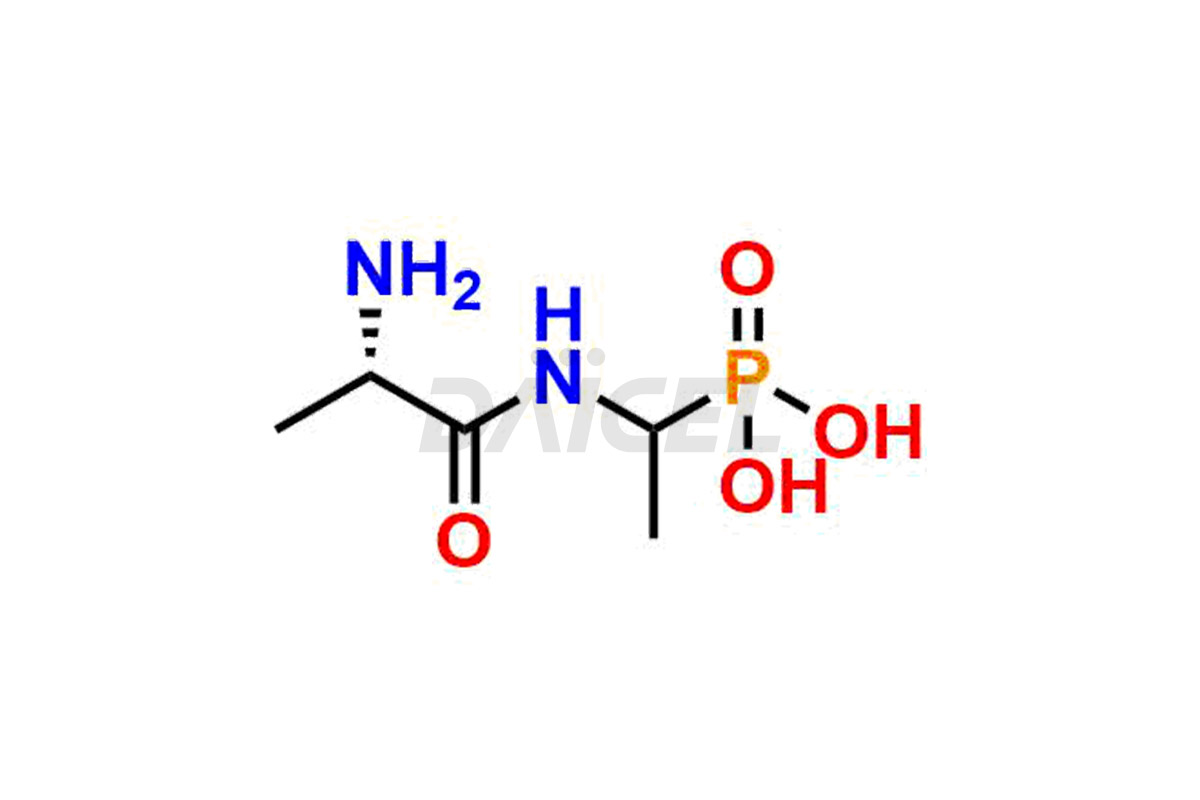
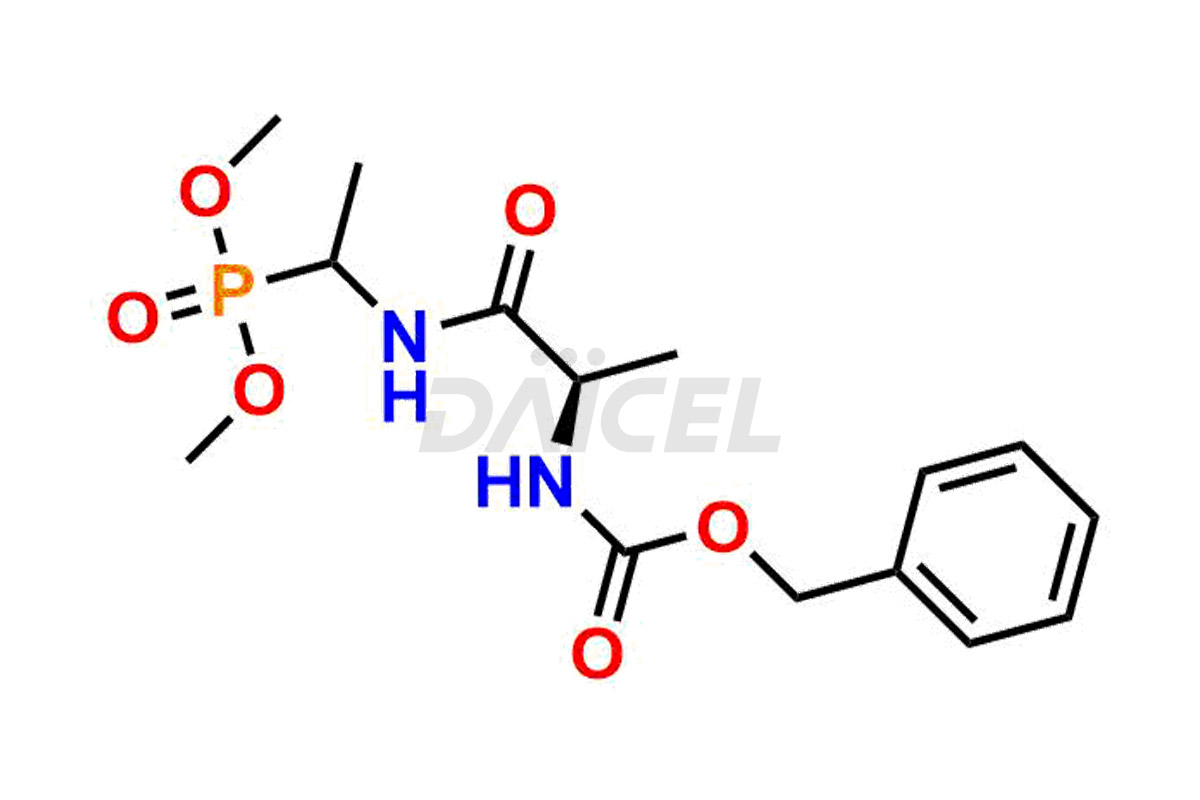
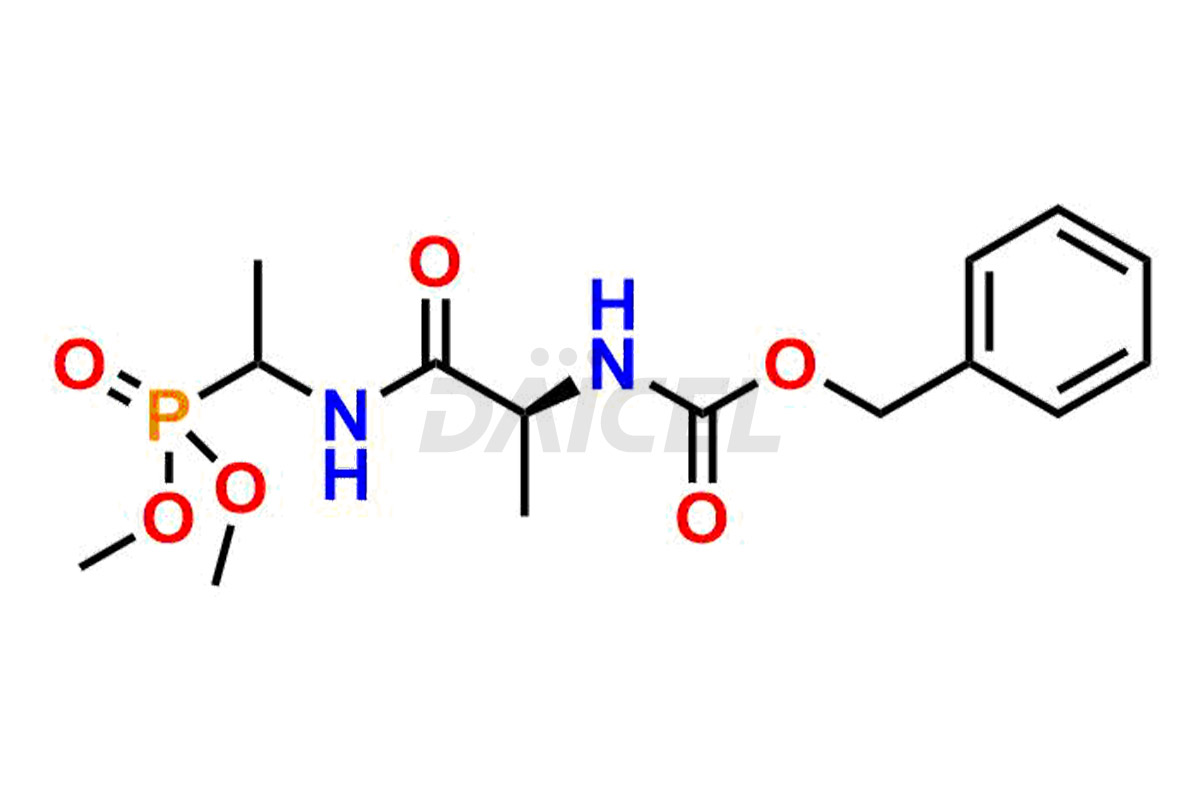
Daicel Pharma is a reliable partner for synthesizing high-quality Alafosfalin impurities, specifically ((S)-1-((S)-2-aminopropanamido)ethyl)phosphonic acid, (1-((S)-2-aminopropanamido)ethyl)phosphonic acid, benzyl ((2R)-1-((1-(dimethoxyphosphoryl)ethyl)amino)-1-oxopropan-2-yl)carbamate, benzyl ((2S)-1-((1-(dimethoxyphosphoryl)ethyl)amino)-1-oxopropan-2-yl)carbamate and L-Alanyl-L-1-aminoethylphosponic acid. These impurities help assess the quality, stability, and safety of the active pharmaceutical ingredient, Alafosfalin. Daicel Pharma also offers a custom synthesis of Alafosfalin impurities, which can be shipped worldwide to meet customers’ unique requirements.
Alafosfalin [CAS: 60668-24-8] is a phosphonodipeptide that inhibits bacterial cell wall biosynthesis. It is an antibacterial, effective against many organisms, particularly Gram-negative bacteria.
Alafosfalin: Use and Commercial Availability
Alafosfalin is a well-known antibacterial agent synthesized by Roche. It has a broad spectrum of antibacterial activity against certain Gram-positive aerobes, anaerobic bacteria, and many species of Enterobacterales. It is highly effective against Escherichia coli and moderately effective against Serratia, Klebsiella, Enterobacter, and Citrobacter. It is bactericidal against Gram-negative bacteria, causing rapid lysis of susceptible strains.
Alafosfalin Structure and Mechanism of Action 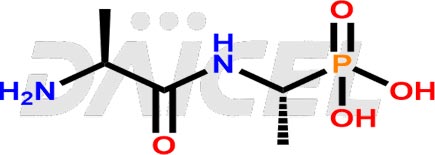
The chemical name of Alafosfalin is (1R)-1-(L-Alanylamino)ethylphosphonic acid. Its chemical formula is C5H13N2O4P, and its molecular weight is approximately 196.14 g/mol.
Alafosfalin bacterial uptake is through LL-dipeptide permeases. Its subsequent hydrolysis yields fosfalin (L-1-aminoethylphosphonic acid). It binds with alanine racemase. Further, it prevents the synthesis of D-alanine, which is essential for peptidoglycan biosynthesis.
Alafosfalin Impurities and Synthesis
There is limited information available on the impurities of Alafosfalin, an antibiotic. However, impurities may form during the synthesis1, storage, and handling of the organic compound. They can impact the efficacy and safety of the drug product. Thus, it is essential to control and minimize impurities during the manufacturing process and ensure appropriate storage conditions to maintain the quality of the drug product.
Daicel Pharma issues a Certificate of Analysis (CoA) for Alafosfalin impurity standards, including ((S)-1-((S)-2-aminopropanamido)ethyl)phosphonic acid, (1-((S)-2-aminopropanamido)ethyl)phosphonic acid, benzyl ((2R)-1-((1-(dimethoxyphosphoryl)ethyl)amino)-1-oxopropan-2-yl)carbamate, benzyl ((2S)-1-((1-(dimethoxyphosphoryl)ethyl)amino)-1-oxopropan-2-yl)carbamate and L-Alanyl-L-1aminoethylphosponic acid. The CoA is from an analytical facility that complies with current Good Manufacturing Practices (cGMP) and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can prepare unknown Alafosfalin impurities or degradation products. A complete characterization report accompanies each delivery.
References
FAQ's
References
- Atherton, Frank R.; Hall, Michael John; Hassall, Cedric H.; Lambert, Robert W.; Ringrose, Peter S., Peptide Derivatives Of Phosphonic And Phosphinic Acids, Hoffmann-La Roche, F., und Co., A.-G., Switzerland, GB1533240A, November 22, 1978
- Galushko, S. V.; Belik, M. Yu.; Solodenko, V. A.; Kasheva, T. N.; Kukhar, V. P., Ion-exchange high-performance liquid chromatography of diastereoisomers of some phosphonodipeptides, Journal of Chromatography, Volume: 600, Issue: 1, Pages: 79-81, 1992
Frequently Asked Questions
What is the role of impurity standards in the analysis of Alafosfalin?
Impurity standards help calibrate and validate analytical methods for detecting impurities in Alafosfalin, ensuring the accuracy and precision of the results.
What is the process for identifying and characterizing unknown Alafosfalin impurities?
Identifying and characterizing unknown Alafosfalin impurities involves many analytical techniques, including HPLC, MS, etc., to determine their chemical structures and toxicity.
Which solvent helps in the analysis of Alafosfalin impurities?
Water or Acetonitrile is a solvent used in analyzing many impurities in Alafosfalin.
What are the temperature conditions required to store Alafosfalin impurities?
Alafosfalin impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.