Afatinib
General Information
Afatinib Impurities and Afatinib
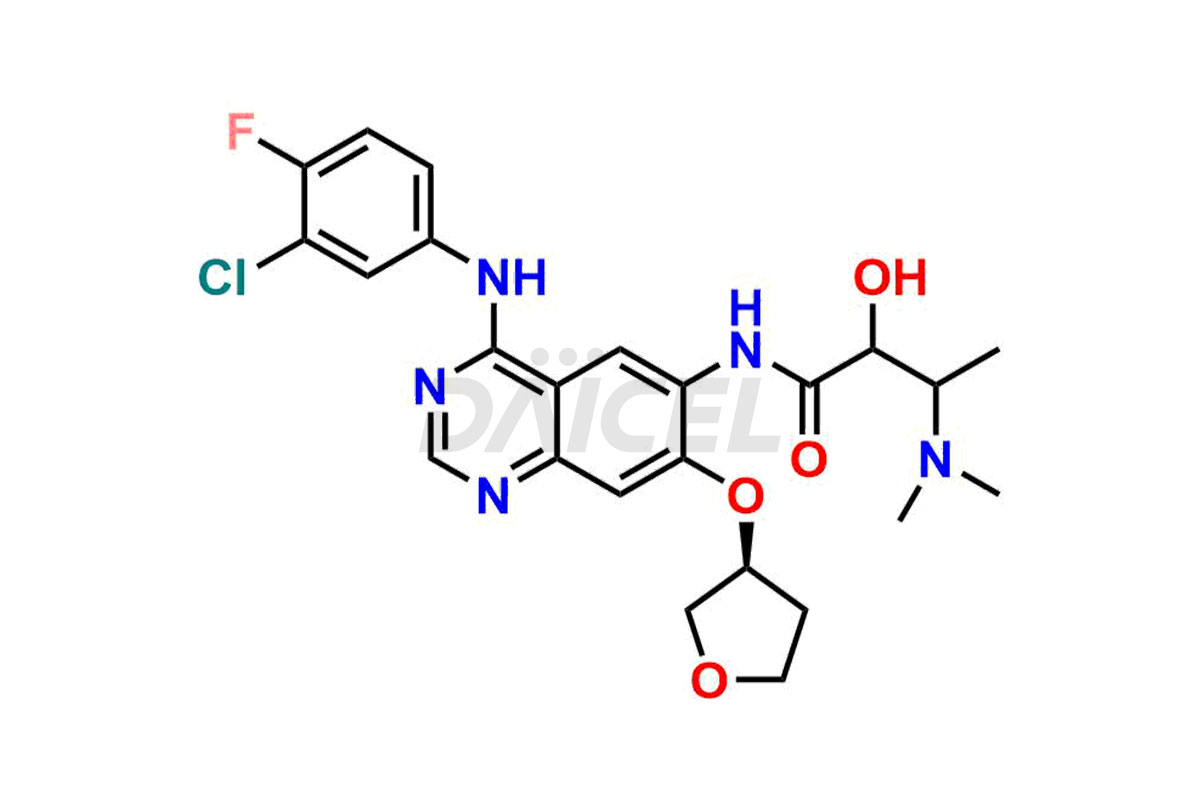
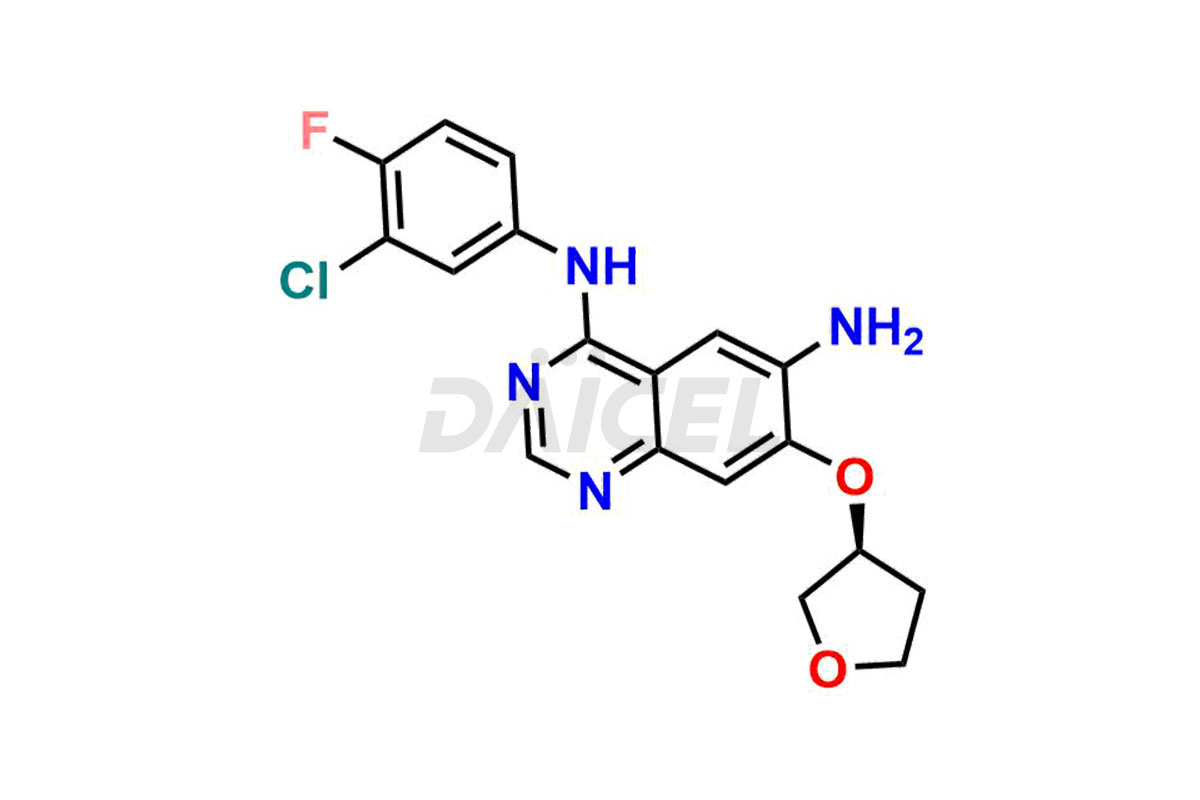
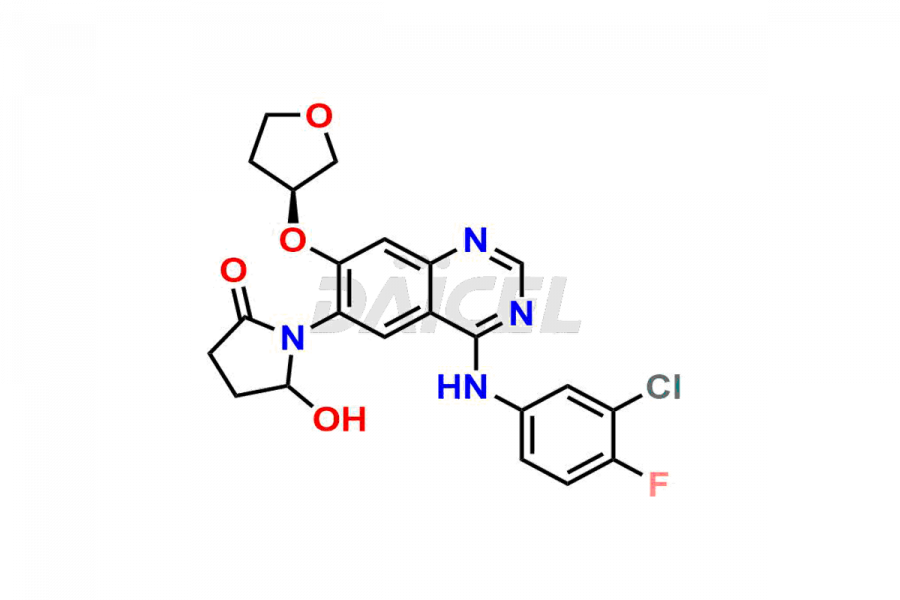
Daicel Pharma synthesizes high-quality Afatinib impurities like 2-Hydroxy 3-dimethylamino Afatinib Impurity, 4-Hydroxy 4-Dedimethylamino Afatinib, Afatinib Bis-dimethylamino Impurity, Afatinib Impurity B, Afatinib Impurity E, and N-(4-((3-chloro-4-fluorophenyl)amino)-7-(((S)-tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-4-(dimethylamino)-3-hydroxybutanamide, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Afatinib. Moreover, Daicel Pharma offers custom synthesis of Afatinib impurities and delivers them globally.
Afatinib [CAS: 850140-72-6] is a medicine of the anilino-quinazoline derivative class of drugs. It inhibits the activity of receptor tyrosine kinases (RTKs) known as the epidermal growth factor receptor (EGFR) family and has antineoplastic activity. Afatinib treats certain types of metastatic non-small cell lung cancer.
Afatinib: Use and Commercial Availability
Afatinib is a medicine that targets and inhibits the ErbB family of tyrosine kinases irreversibly. The FDA has approved its use as a first-line treatment for locally advanced or metastatic non-small cell lung cancer (NSCLC) with non-resistant epidermal growth factor receptor (EGFR) mutations. Additionally, it acts as a second-line treatment for advanced squamous non-small cell lung cancer (NSCLC). Gilotrif is the trade name for Afatinib.
Afatinib Structure and Mechanism of Action 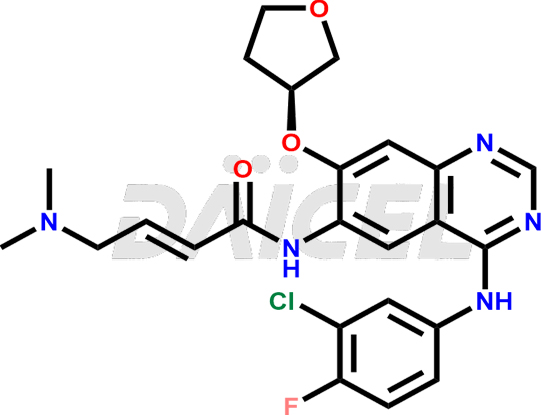
The chemical name of Afatinib is (2E)-N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butenamide. Its chemical formula is C24H25ClFN5O3, and its molecular weight is approximately 485.9 g/mol.
Afatinib binds to kinase domains, EGFR (ErbB1), HER2 (ErbB2), and HER4 (ErbB4). It inhibits tyrosine kinase autophosphorylation leading to the down-regulation of ErbB signaling.
Afatinib Impurities and Their Synthesis
During synthesis, storage, and use of Afatinib, impurities may arise, including organic impurities, such as intermediates, starting materials and potential genotoxic impurities, and inorganic impurities. Their presence compromises the safety and efficacy of the medication, making it crucial to control them. Strict quality control measures such as regular monitoring and testing are necessary to detect and manage Afatinib impurities.
Daicel provides a Certificate of Analysis (CoA) for Afatinib impurity standards, including 2-Hydroxy 3-dimethylamino Afatinib Impurity, 4-Hydroxy 4-Dedimethylamino Afatinib, Afatinib Bis-dimethylamino Impurity, Afatinib Impurity B, Afatinib Impurity E, and N-(4-((3-chloro-4-fluorophenyl)amino)-7-(((S)-tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-4-(dimethylamino)-3-hydroxybutanamide. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Afatinib impurity or degradation product and offer labeled compounds to quantify the efficacy of Afatinib. Daicel offers Afatinib-D6, a deuterium-labeled Afatinib standard used in bio-analytical research such as BA/BE studies. We give a complete characterization report on delivery.
References
FAQ's
References
- Soyka, Rainer; Rall, Werner; Schnaubelt, Juergen; Sieger, Peter; Kulinna, Christian, Process for preparing amino crotonyl compounds, Boehringer Ingelheim International GmbH, Germany, US8426586B2, April 25, 2013
- Ankalla, Kiran Kumar; Gollapalli, Nageswara Rao, A stability-indicating UPLC method for quantification of Afatinib related substances in pharmaceutical dosage forms, World Journal of Pharmacy and Pharmaceutical Sciences, Volume: 6, Issue: 12, Pages: 1253-1267, 2017
Frequently Asked Questions
How are the impurities formed in Afatinib?
Impurities in Afatinib occur from raw materials, incomplete reaction, degradation during storage, and purification.
Do impurities in Afatinib affect the pharmacokinetics of the drug?
The impurities in Afatinib affect the pharmacokinetics leading to changes in bioavailability, metabolism, and elimination.
Which solvent helps in the analysis of Afatinib impurities?
Methanol is a solvent used in analyzing many impurities in Afatinib.
What are the temperature conditions required to store Afatinib impurities?
Afatinib impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.