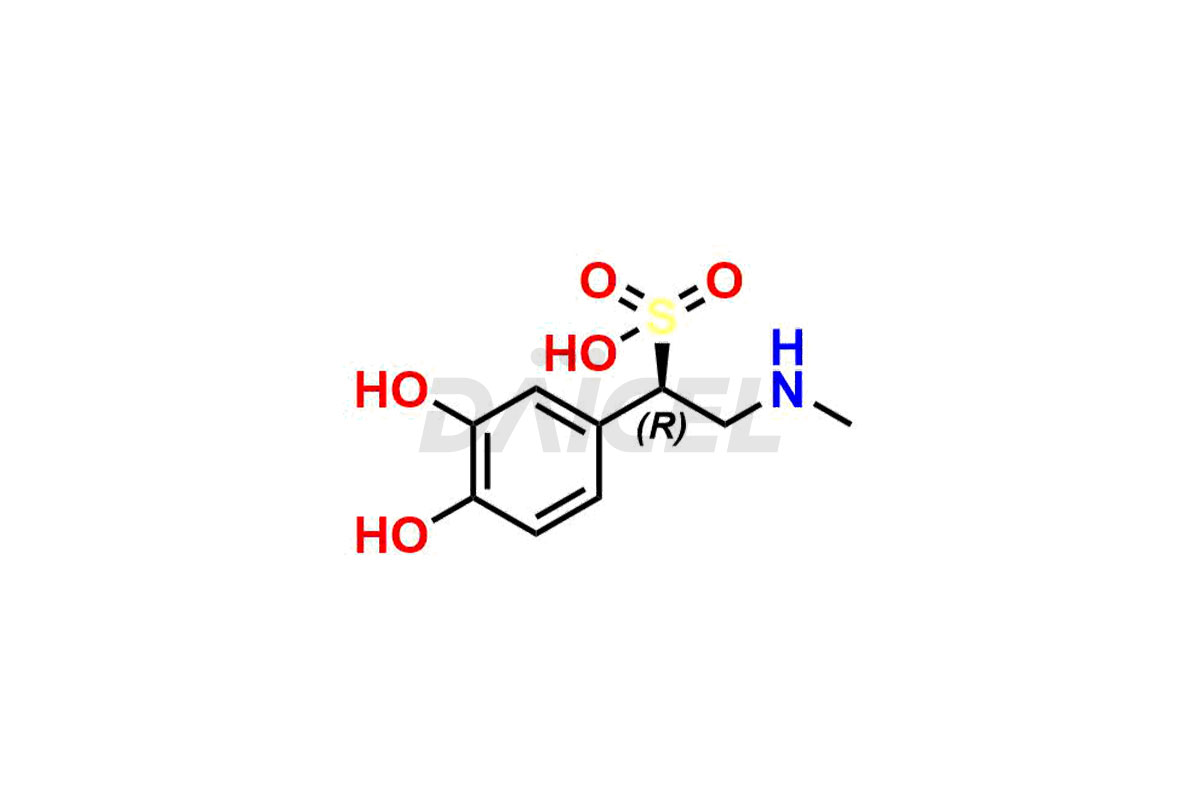
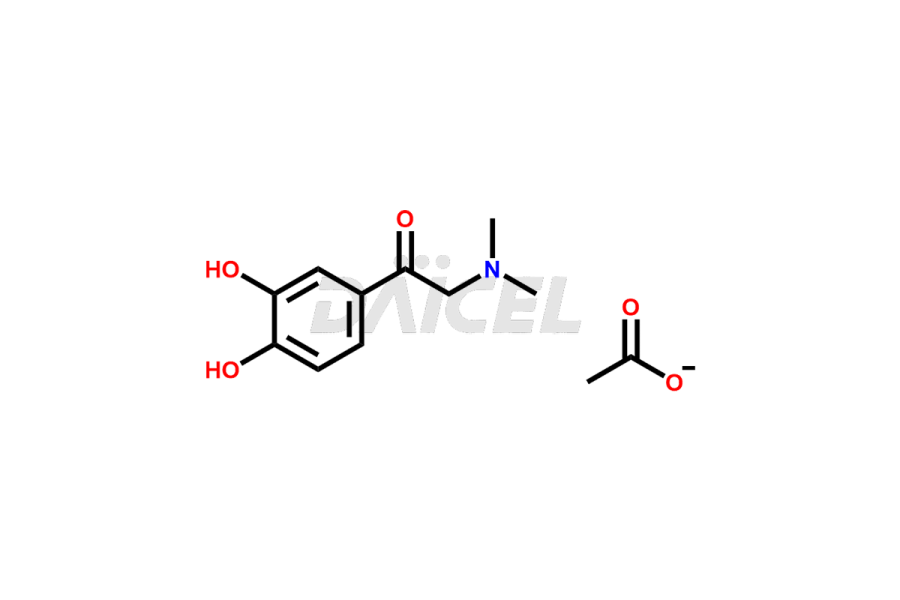
Adrenaline
General Information
Adrenaline Impurities and Adrenaline
Daicel Pharma synthesizes high-quality Adrenaline impurity, Adrenaline Impurity – F, which is crucial in analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient Adrenaline. Moreover, Daicel Pharma offers custom synthesis of Adrenaline impurities and delivers them globally.
Adrenaline [CAS: 51-43-4], or therapeutic Epinephrine, is a synthetic derivative of the endogenous sympathomimetic amine. It has vasoconstrictive, bronchodilatory, and intraocular pressure-lowering activities. Epinephrine (Adrenaline) is a hormone and neurotransmitter produced by adrenal glands. It is for emergency treatment of severe allergic reactions.
Adrenaline: Use and Commercial Availability
Adrenaline is a widely used medicine that treats severe allergic reactions (anaphylaxis), maintains pupil dilation during eye surgeries, and treats septic shock-induced hypotension. Adrenaline is used as a local anesthetic. It acts on the body’s stress response by stimulating the sympathetic nervous system. There are many brand names for Adrenaline, including Adrenaclick, Auvi-Q, Duranest, Epipen, etc.
Adrenaline Structure and Mechanism of Action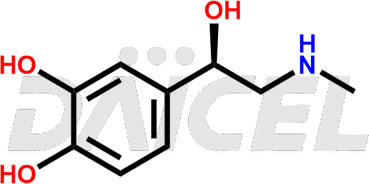
The chemical name of Adrenaline is 4-[(1R)-1-Hydroxy-2-(methylamino)ethyl]-1,2-benzenediol. Its chemical formula is C9H13NO3, and its molecular weight is approximately 183.20 g/mol.
Adrenaline is a sympathomimetic acting on alpha and beta receptors. Its vasoconstrictor action on alpha receptors increases vascular permeability leading to loss of intravascular fluid volume and hypotension during anaphylactic reactions. Further, its action on beta receptors causes bronchial smooth muscle relaxation that alleviates wheezing.
Adrenaline Impurities and Synthesis
Impurities form during manufacturing1, storage, or use of Adrenaline. These can be organic such as process-related or degradation products, or inorganic. It is necessary to control impurities to ensure the safety and efficacy of the medicine with regular testing and monitoring.
Daicel provides a Certificate of Analysis (CoA) for the Adrenaline impurity standard, Adrenaline Impurity – F. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data2,3, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Adrenaline impurity or degradation product. We give a complete characterization report on delivery.
References
FAQ's
References
- Jowett, Hooper Albert Dickinson, The constitution of epinephrine, Journal of the Chemical Society, Transactions, Volume: 85, Pages: 192-197, 1904
- Kojima, Ayako, An automated method for the determination of adrenaline and noradrenaline, Industrial Health, Volume: 4, Issue: 3, Pages: 98-108, 1966
- Choulis, Nicolas H., Separation and determination of adrenaline using thin-layer chromatography, Journal of Pharmaceutical Sciences, Volume: 56, Issue: 2, Pages: 196-9, 1967
Frequently Asked Questions
Do impurities in Adrenaline affect its potency?
The impurities can affect the potency of Adrenaline, potentially reducing its effectiveness in treating some conditions.
Does the presence of impurities affect the shelf life of Adrenaline?
Impurities can accelerate the degradation of Adrenaline, leading to shorter shelf life.
Which solvent helps in the analysis of Adrenaline impurities?
Water is the solvent used in analyzing many impurities in Adrenaline.
What are the temperature conditions required to store Adrenaline impurities?
Adrenaline impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.