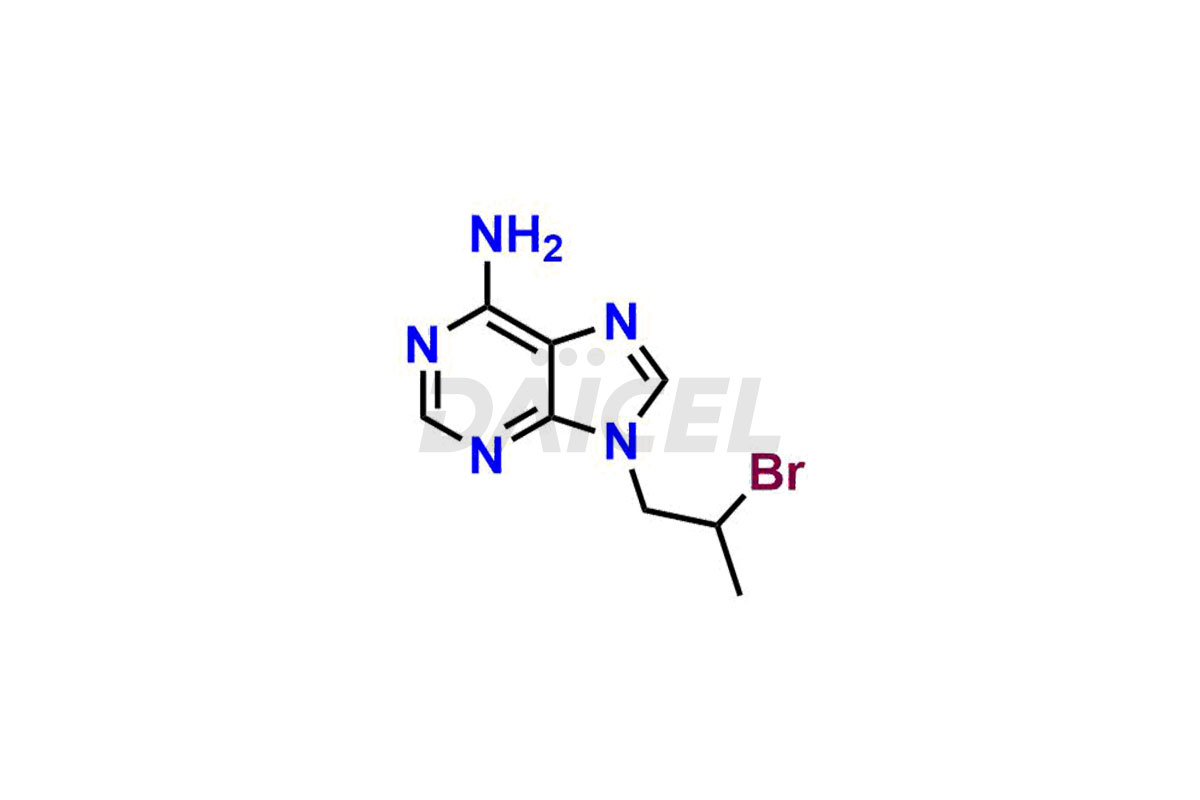
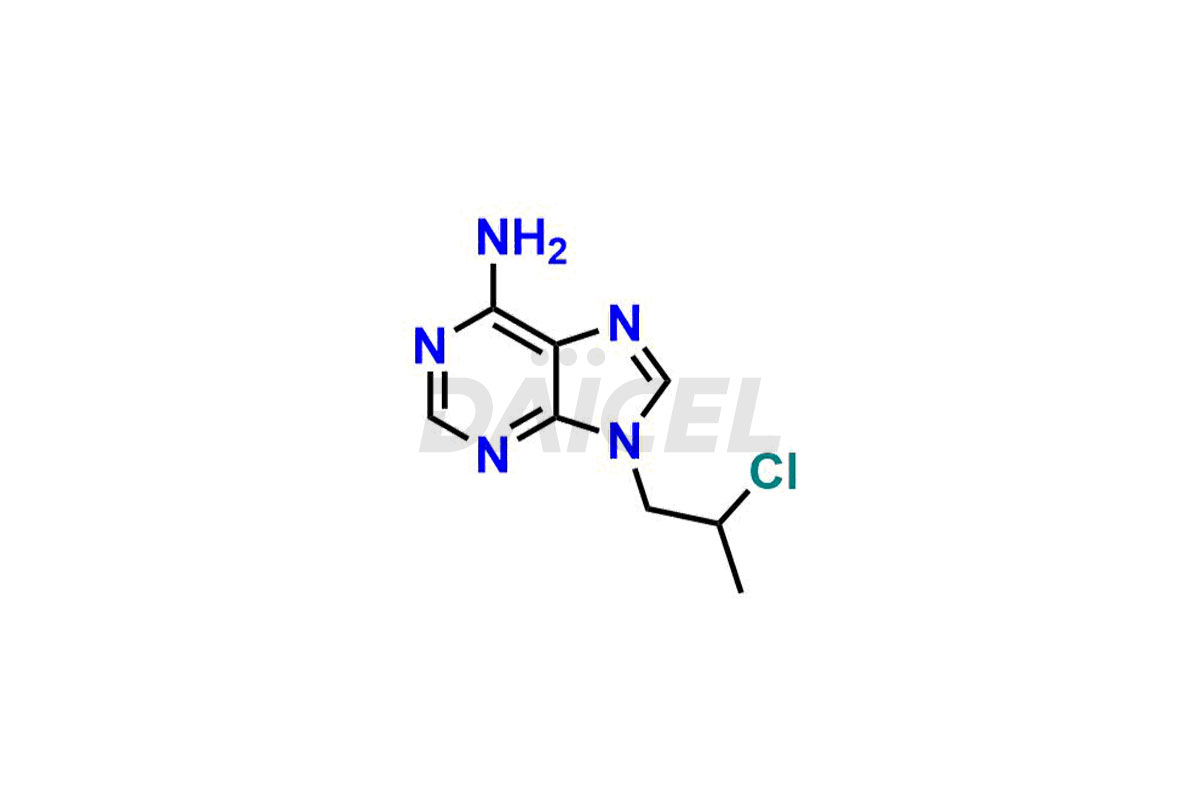
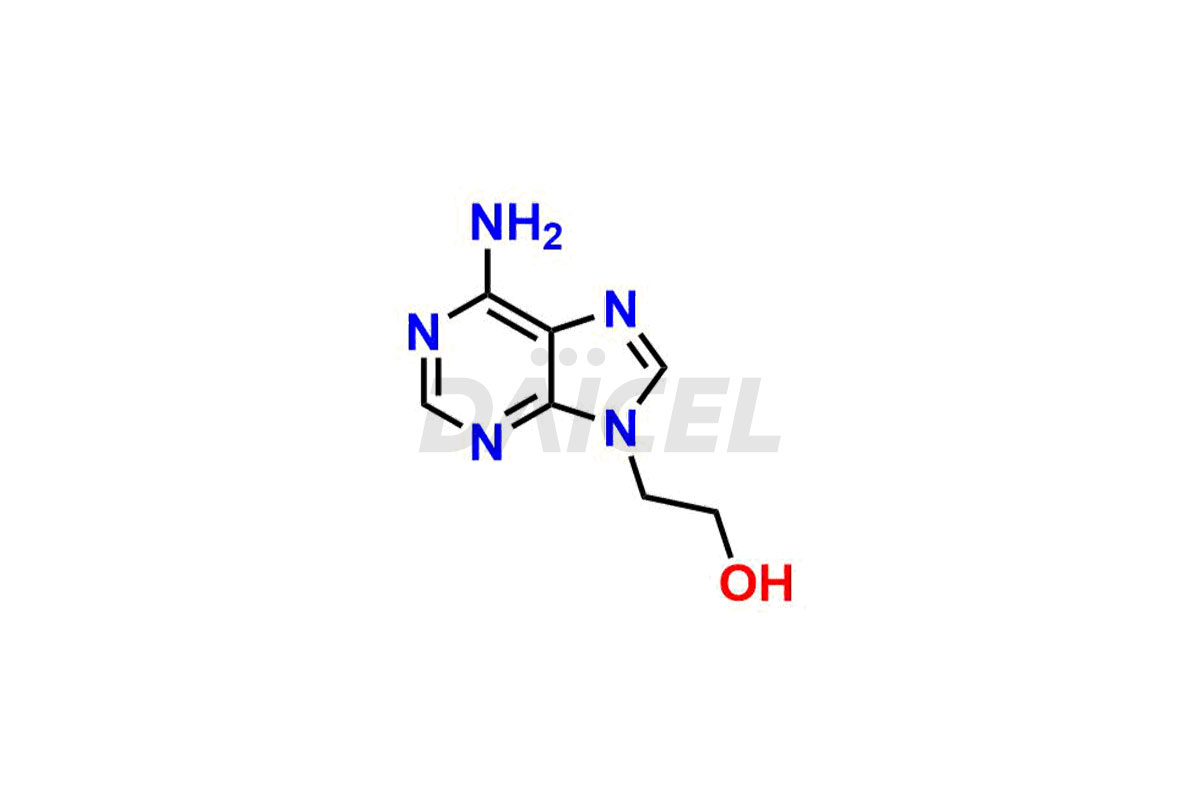
Adenine
General Information
Adenine Impurities and Adenine
Daicel Pharma synthesizes high-quality Adenine impurities like 2-Bromopropyl Adenine, 2-Chloropropyl Adenine, and 9-(2-Hydroxy ethyl) Adenine, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Adenine. Moreover, Daicel Pharma offers custom synthesis of Adenine impurities and delivers them globally.
Adenine [CAS: 73-24-5] is a purine nucleobase belonging to the class of 6-aminopurines. Adenine is the precursor of two important nucleosides, Adenosine, and deoxyadenosine. These nucleosides are crucial building blocks of DNA and RNA molecules, essential for the proper functioning of cells in living organisms.
Adenine: Use and Commercial Availability
Adenine is a purine base organic compound that is an essential component of DNA and RNA. It is also present in many multivitamins and is used for nutritional supplementation to address dietary deficiencies or imbalances. Adenine is an active ingredient in many products, including Rejuvesol and CPDA-1.
Adenine Structure and Mechanism of Action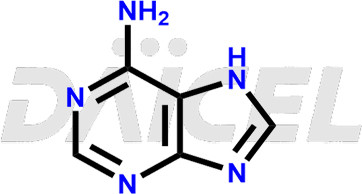
The chemical name of Adenine is 9H-Purin-6-amine. Its chemical formula is C5H5N5, and its molecular weight is approximately 135.13 g/mol.
Adenine is a crucial component of Adenine nucleotides and forms Adenosine, a nucleoside when attached to ribose, and deoxyadenosine when attached to deoxyribose. It forms Adenosine triphosphate (ATP) when three phosphate groups add to Adenosine, and it helps in cellular metabolism by transferring chemical energy between chemical reactions.
Adenine Impurities and Synthesis
The impurities in Adenine are classified as organic and inorganic impurities. Organic impurities may include related substances such as Adenine analogs, while inorganic impurities may include heavy metals or other contaminants introduced during synthesis1,2. Impurities can form during the synthesis, isolation, and purification of Adenine. The analysis and control of these impurities are essential to ensure that the final product meets the required quality standards for its intended use, whether for research, pharmaceuticals, or other applications.
Daicel provides a Certificate of Analysis (CoA) for Adenine impurity standards, including 2-Bromopropyl Adenine, 2-Chloropropyl Adenine, and 9-(2-Hydroxy ethyl) Adenine. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data3, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Adenine impurity or degradation product. We give a complete characterization report on delivery.
References
FAQ's
References
- Ichikawa, Takehiko; Kato, Tetsuya; Takenishi, Tadao, New synthesis of adenine and 4-aminoimidazole-5-carboxamide, Journal of Heterocyclic Chemistry, Volume: 2. Issue: 3, Pages: 253-5, 1965
- Ochiai, M.; Marumoto, R.; Kobayashi, S.; Shimazu, H.; Morita, K., A facile one-step synthesis of adenine, Tetrahedron, Volume: 24, Issue: 17, Pages: 5731-7, 1968
- Remenchik, Alexander P.; Bernsohn, Joseph, Thin-layer chromatographic separation of adenine nucleotides from erythrocytes, Analytical Biochemistry, Volume: 18, Issue: 1, Pages: 1-9, 1967
Frequently Asked Questions
How are the Adenine impurities monitored during the manufacturing process?
Impurities in Adenine are monitored through regular quality control testing using analytical methods such as HPLC and LC-MS.
Why is it essential to control Adenine impurities?
Impurities in Adenine affect the drug’s purity, potency, and stability. They can also impact the quality and effectiveness of products made using Adenine.
How are the impurities in Adenine removed?
Impurities in Adenine can be separated through various purification techniques such as chromatography, and recrystallization.
What are the temperature conditions required to store Adenine impurities?
Adenine impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.