Acyclovir
General Information
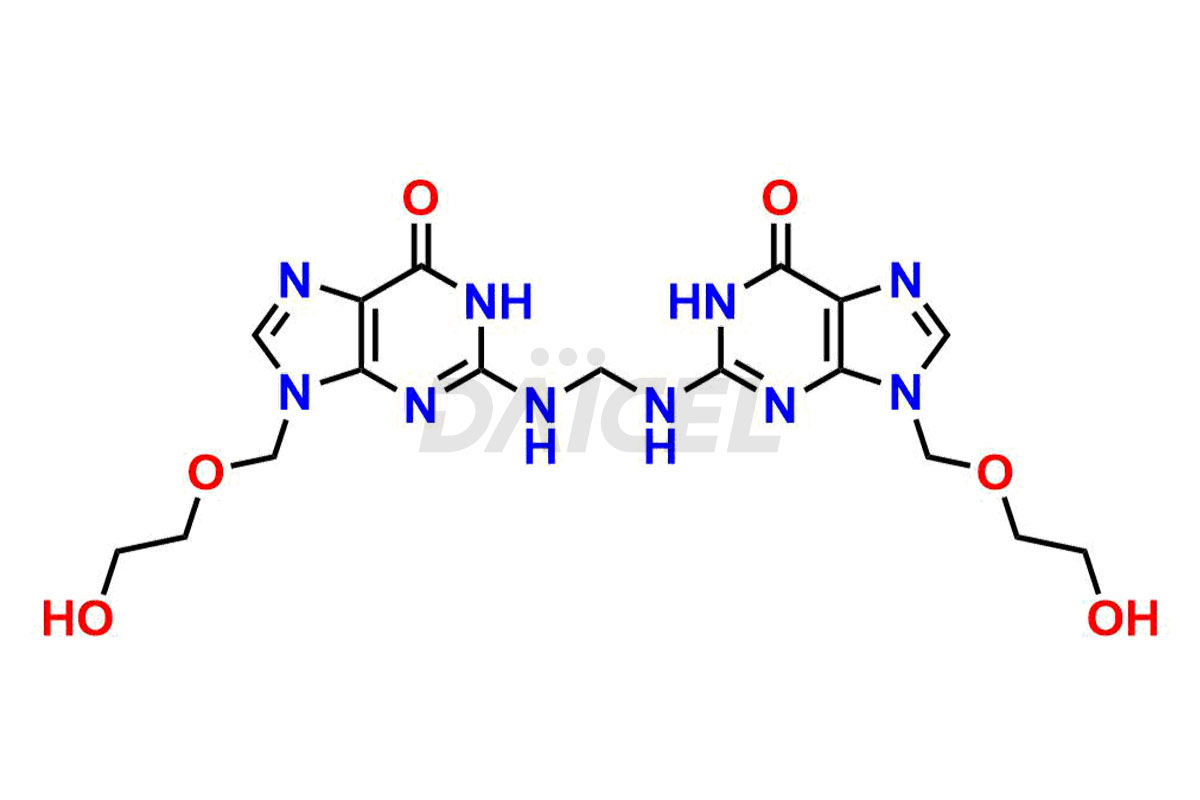
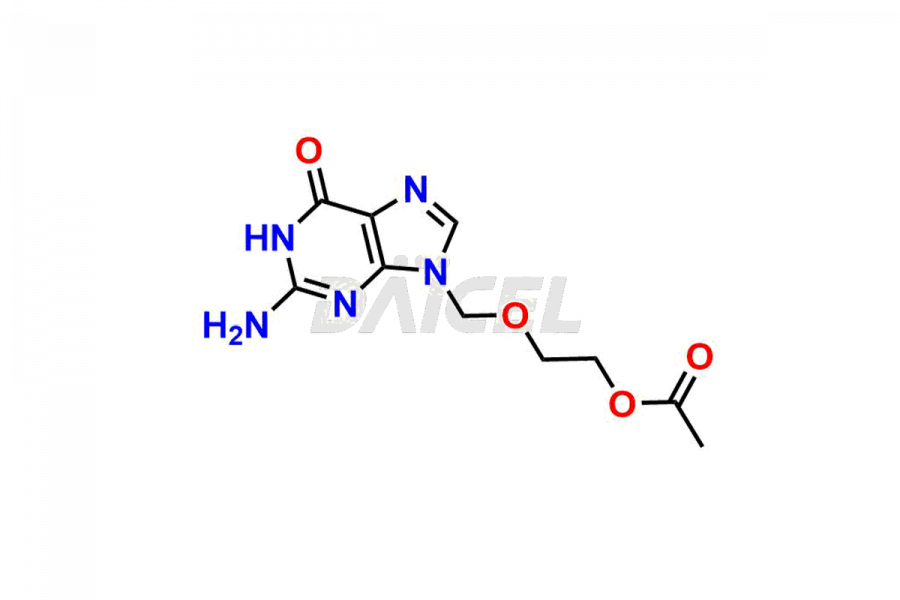
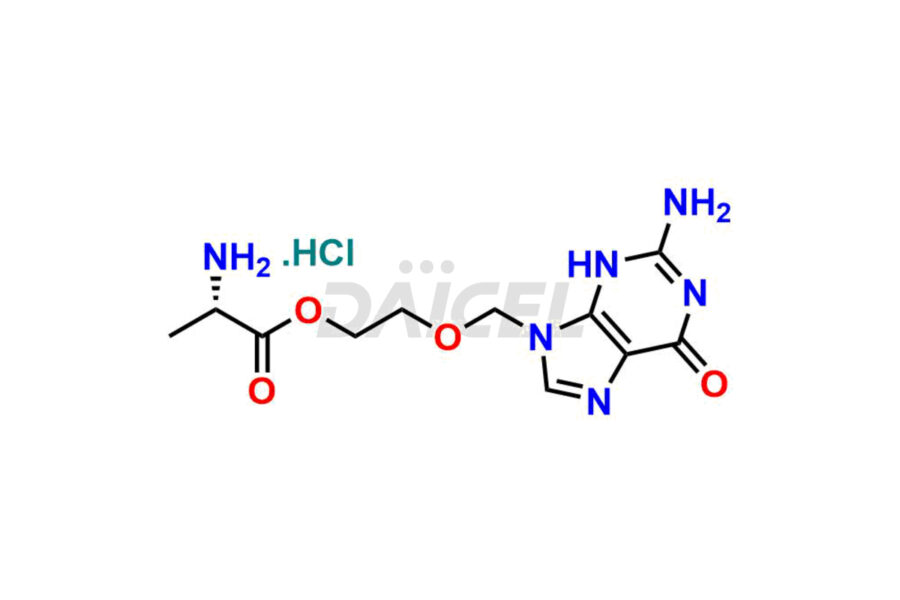
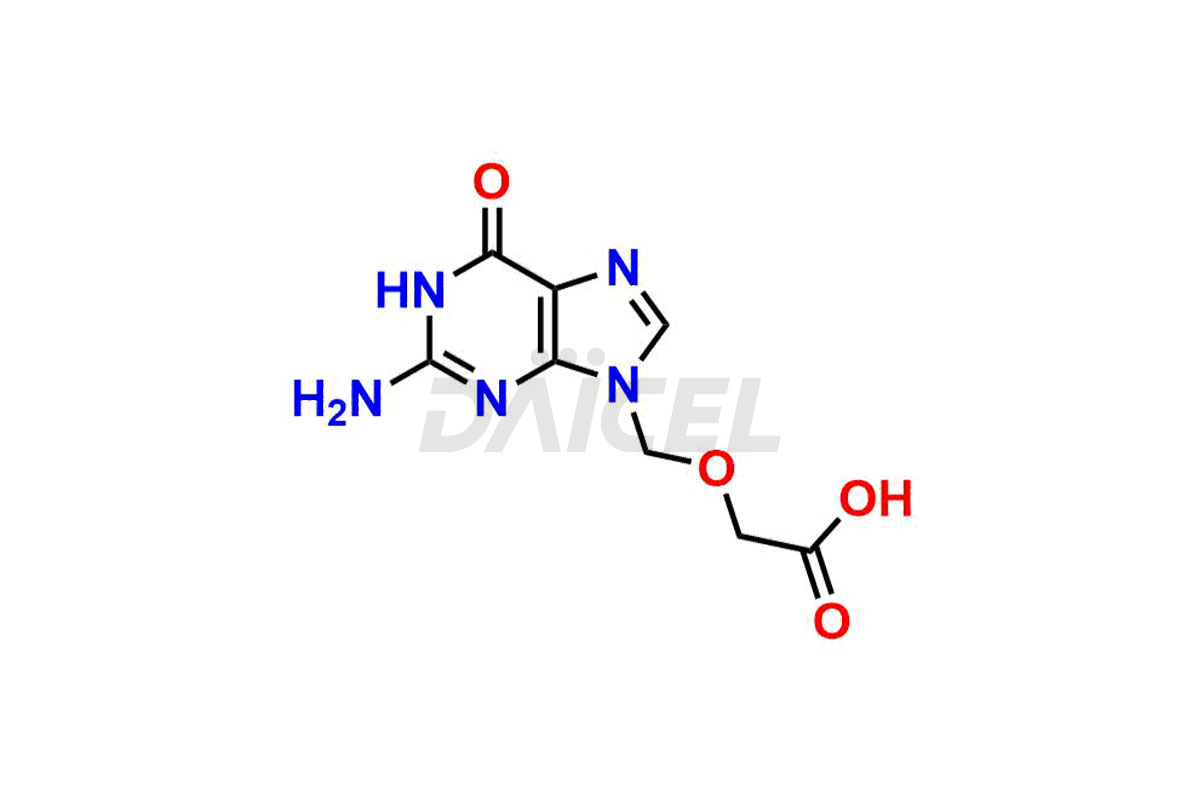
Acyclovir Impurities and Acyclovir
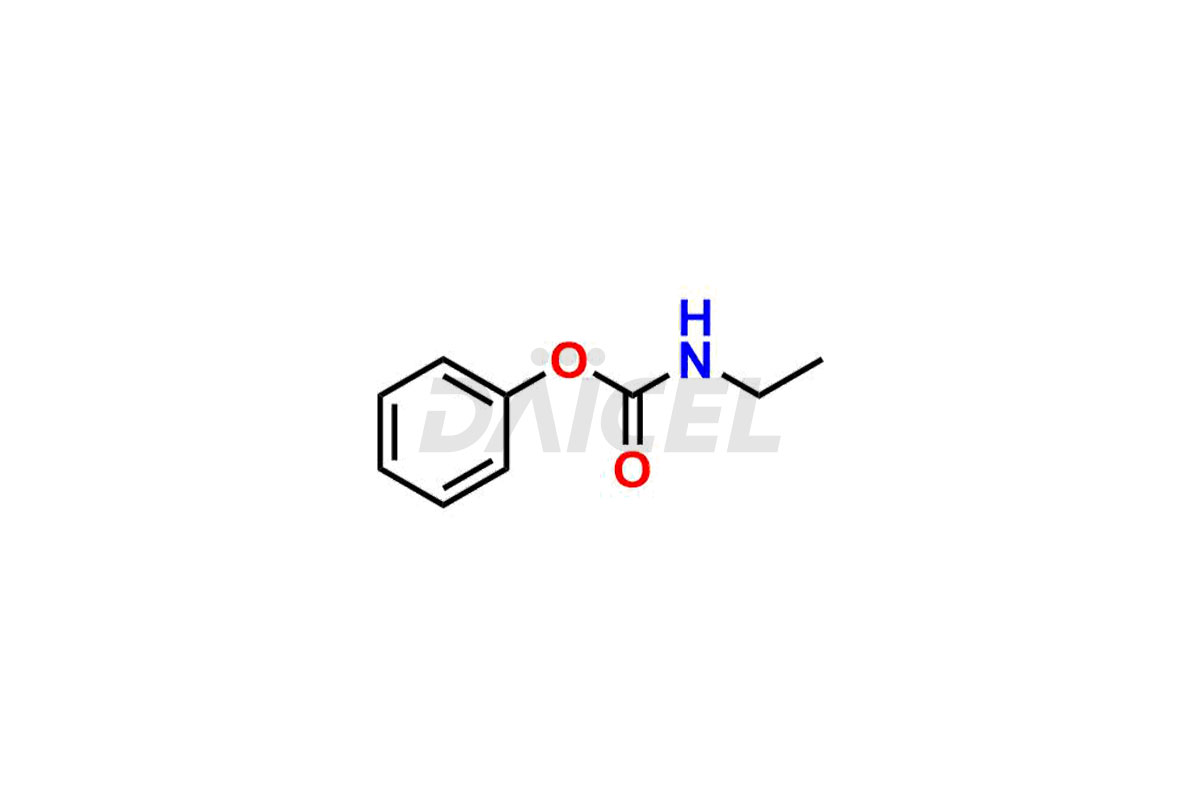
Daicel Pharma synthesizes high-quality Acyclovir impurities like Acyclovir Impurity A, Acyclovir EP Impurity-K, 2-(acetyloxymethoxy)ethoxymethyl acetate, Phenyl N-ethyl Carbamate, Carboxy Acyclovir Imp INH, and Acyclovir L-Alaninate, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Acyclovir. Moreover, Daicel Pharma offers custom synthesis of Acyclovir impurities and delivers them globally.
Acyclovir [CAS: 59277-89-3] is a synthetic analog of the purine nucleoside guanosine. It is effective against the herpes simplex virus types 1 and 2, the varicella-zoster virus, and other viruses in the herpes family. Acyclovir also treats genital herpes and HSV encephalitis.
Acyclovir: Use and Commercial Availability
Acyclovir is available in various dosage forms depending on the patient’s condition. As a topical cream, Acyclovir treats recurrent herpes labialis in patients. Suspensions, capsules, and oral tablets of Acyclovir treat chickenpox, herpes zoster, and genital herpes. Acyclovir buccal tablet treats recurrent herpes labialis. Acyclovir topical ointment treats initial genital herpes and limits non-life-threatening mucocutaneous herpes simplex in patients with a weakened immune system. Acyclovir ophthalmic ointment treats acute herpetic keratitis. Acyclovir is available under various trade names, including Avaclyr, Sitavig, Xerese, and Zovirax.
Acyclovir Structure and Mechanism of Action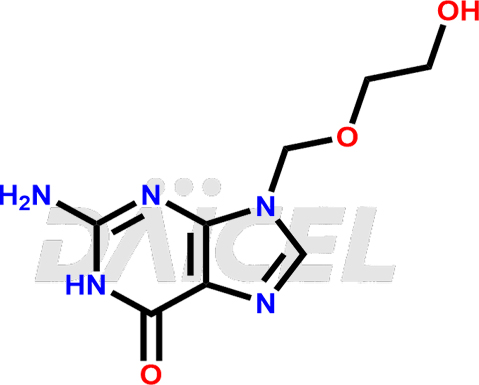
The chemical name of Acyclovir is 2-Amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one. Its chemical formula is C8H11N5O3, and its molecular weight is approximately 225.20 g/mol.
Acyclovir inhibits the activity of herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV). The inhibitory activity is due to its affinity for the thymidine kinase (TK) enzyme.
Acyclovir Impurities and Synthesis
Impurities in Acyclovir can be categorized as organic, inorganic, or residual solvents. Organic impurities form during the synthesis or storage of the drug, while inorganic impurities result from the starting materials or reagents. Residual solvents can also be present due to the manufacturing1 process. Controlling these Acyclovir impurities is important as they can affect drug purity, potency, stability, and safety.
Daicel provides a Certificate of Analysis (CoA) for Acyclovir impurity standards, including Acyclovir Impurity A, Acyclovir EP Impurity-K, 2-(acetyloxymethoxy)ethoxymethyl acetate, Phenyl N-ethyl Carbamate, Carboxy Acyclovir Imp INH, and Acyclovir L-Alaninate. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Acyclovir impurity or degradation product. We give a complete characterization report on delivery.
References
FAQ's
References
- Schaeffer, Howard John, Purine Compounds and Salts Thereof, Wellcome Foundation Ltd., United Kingdom, GB1523865A, September 6, 1978
- Land, G.; Bye, A., Simple high-performance liquid chromatographic method for the analysis of 9-(2-hydroxyethoxymethyl)guanine (acyclovir) in human plasma and urine, Journal of Chromatography, Biomedical Applications, Volume: 224, Issue: 1, Pages: 51-8, 1981,
Frequently Asked Questions
Do impurities in Acyclovir vary between different manufacturers?
Acyclovir impurities vary between manufacturers depending on their manufacturing processes, starting materials, and quality control measures.
Do impurities in Acyclovir affect its stability?
The impurities in Acyclovir affect its stability and shelf life. They can accelerate degradation and lead to the formation of other impurities.
What are the different types of Acyclovir impurities?
The different types of impurities in Acyclovir include organic and inorganic impurities, residual solvents, and degradation products.
What are the temperature conditions required to store Acyclovir impurities?
Acyclovir impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.