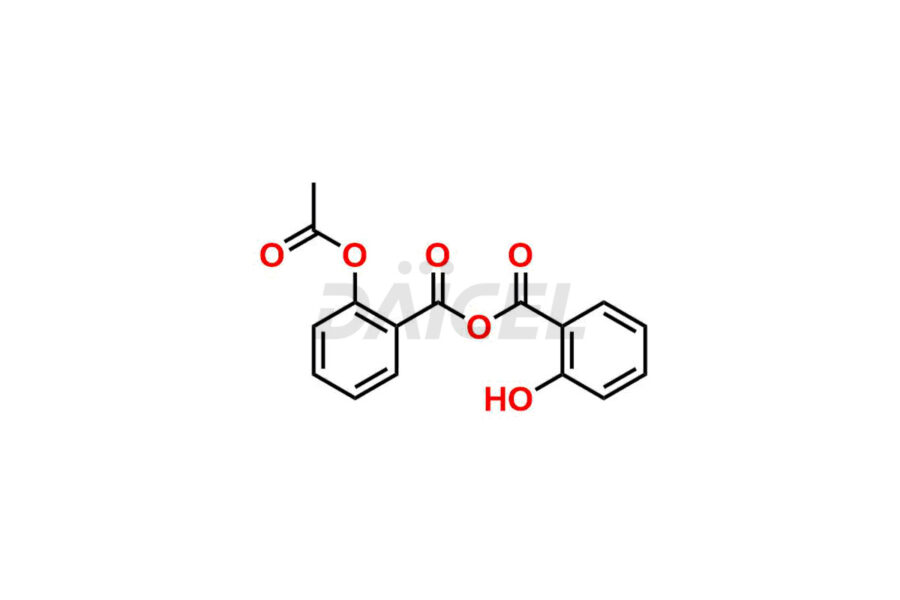
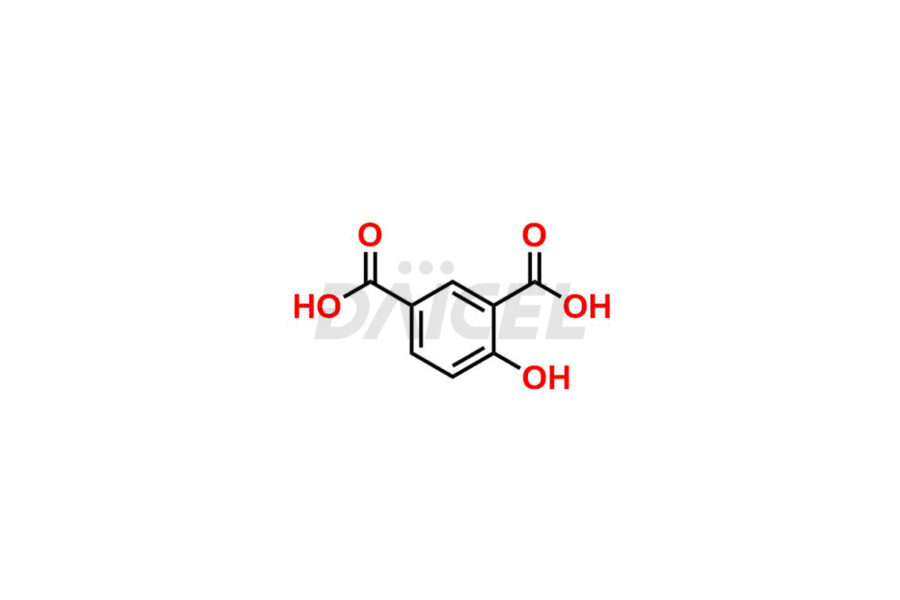
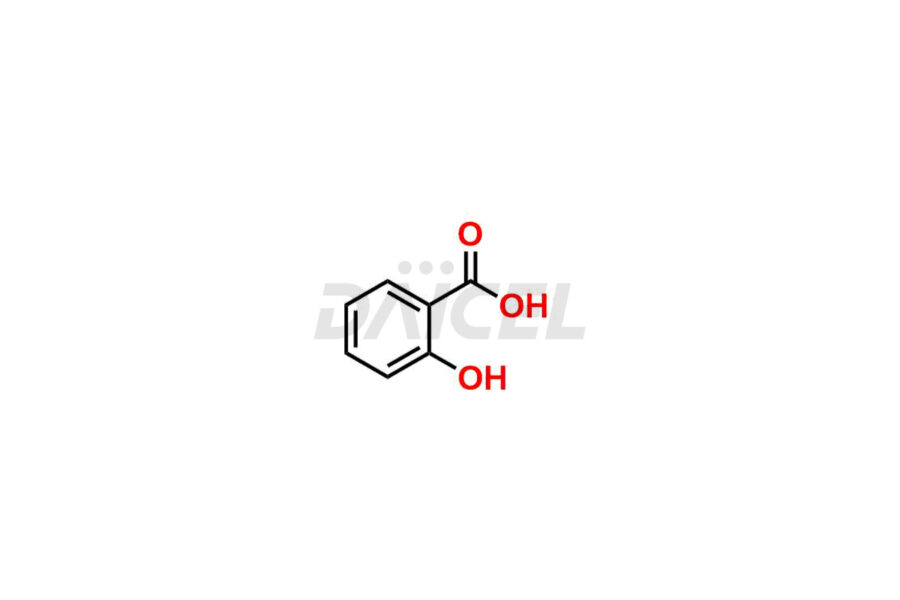
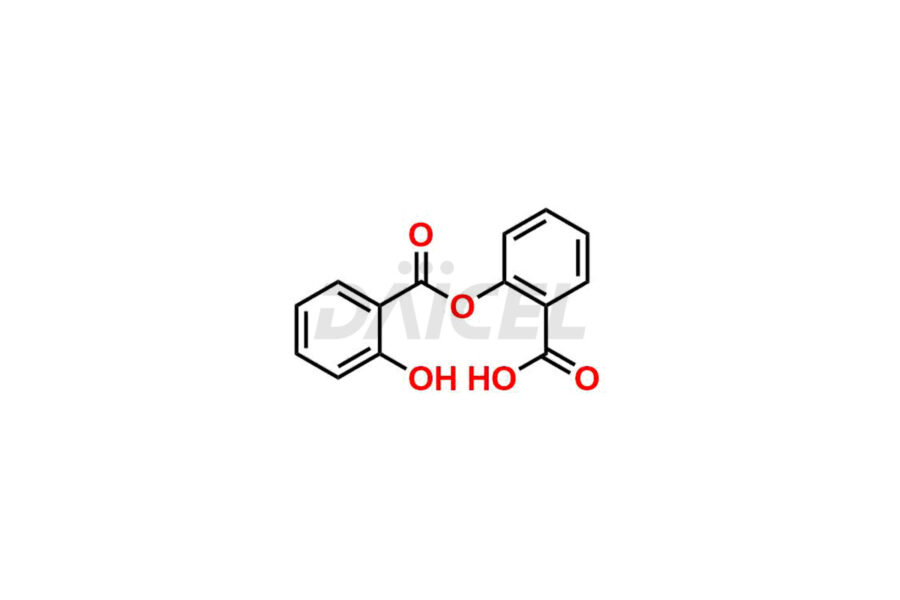
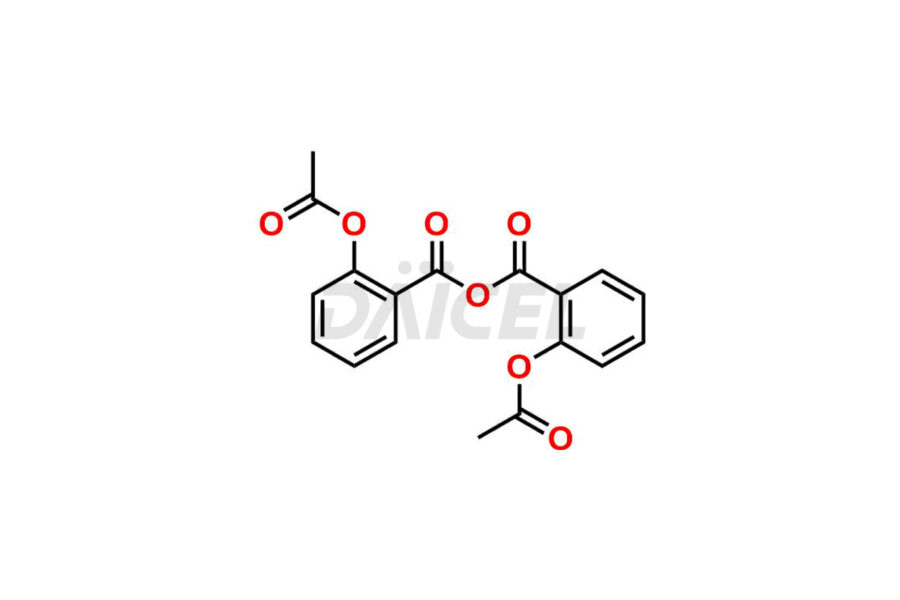
Acetyl salicylic acid
General Information
Acetyl Salicylic Acid Impurities and Acetyl Salicylic Acid
Daicel Pharma synthesizes high-quality Acetyl Salicylic Acid impurities like Acetyl Salicylic Acid EP Impurity-B, Acetyl Salicylic Acid EP Impurity-C, Methyl 5-acetylsalicylate, 2-acetoxybenzoic 2-hydroxybenzoic anhydride, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Acetyl Salicylic Acid. Moreover, Daicel Pharma offers custom synthesis of Acetyl Salicylic Acid impurities and delivers them globally.
Aspirin, also known as acetylsalicylic acid [CAS: 50-78-2], is a widely used analgesic and antipyretic medicine and has been used clinically for a long time. It is an orally administered non-steroidal anti-inflammatory agent with analgesic, antipyretic, and anticoagulant properties.
Acetyl Salicylic Acid: Use and Commercial Availability
Acetylsalicylic acid acts as an analgesic, antipyretic, and anti-inflammatory medicine. Additionally, aspirin has anticoagulant properties and is used in low doses over a long period to prevent heart attacks and cancer. Aspirin treats many conditions, including angina pectoris, ankylosing spondylitis, fever, osteoarthritis, pain, rheumatoid arthritis, and systemic lupus erythematosus, and prevents myocardial infarction, ischemic stroke, and revascularization procedures. The drug is available under many trade names, including Aggrenox, Axotal, Codoxy, Fiorinal, etc.
Acetyl Salicylic Acid Structure and Mechanism of Action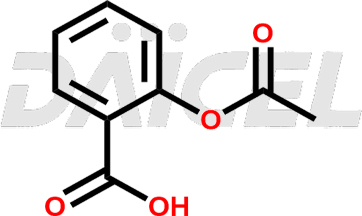
The chemical formula for Acetyl Salicylic Acid (Aspirin) is C9H8O4, and its molecular weight is approximately 180.16 g/mol.
Aspirin inhibits the activity of the cyclooxygenase (COX) enzyme, which leads to prostaglandins formation and causes inflammation, pain, and fever.
Acetyl Salicylic Acid Impurities and Synthesis
Impurities in acetylsalicylic acid arise from various factors such as the quality of the starting materials, synthetic1 processes, and storage conditions. They produce from starting materials, byproducts, or degradation of the drug substance. The formation of these impurities affects the purity and efficacy of the drug product, and hence, it is necessary to monitor and control the levels of acetylsalicylic acid impurities.
Daicel provides a Certificate of Analysis (CoA) for Acetyl Salicylic Acid impurity standards, including Acetyl Salicylic Acid EP Impurity-B, Acetyl Salicylic Acid EP Impurity-C, Methyl 5-acetylsalicylate, 2-acetoxybenzoic 2-hydroxybenzoic anhydride, and more. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Acetyl Salicylic Acid impurity or degradation product. We give a complete characterization report on delivery.
References
FAQ's
References
- Volwiler, E. H.; Vliet, E. B., Preparation and hydrolysis of benzyl esters, Journal of the American Chemical Society, Volume: 43, Pages: 1672-6, 1921
- Rowland, Malcolm; Riegelman, Sidney, Determination of acetylsalicylic acid and salicylic acid in plasma, Journal of Pharmaceutical Sciences, Volume: 56, Issue: 6, Pages: 717-20, 1967
Frequently Asked Questions
How are the Acetyl Salicylic Acid impurities detected?
Impurities in Acetyl Salicylic Acid impurities (Aspirin) are detected using analytical techniques such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), etc.
Are Acetyl Salicylic Acid impurities a common problem?
Impurities in Acetyl Salicylic Acid impurities (Aspirin) are rare, but poor manufacturing practices, improper handling of raw materials, or inadequate quality control measures can generate them.
Which solvent helps in the analysis of Acetyl Salicylic Acid impurities?
Methanol is the solvent used in analyzing many impurities in Acetyl Salicylic Acid.
What are the temperature conditions required to store Acetyl Salicylic Acid impurities?
Acetyl Salicylic Acid impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.