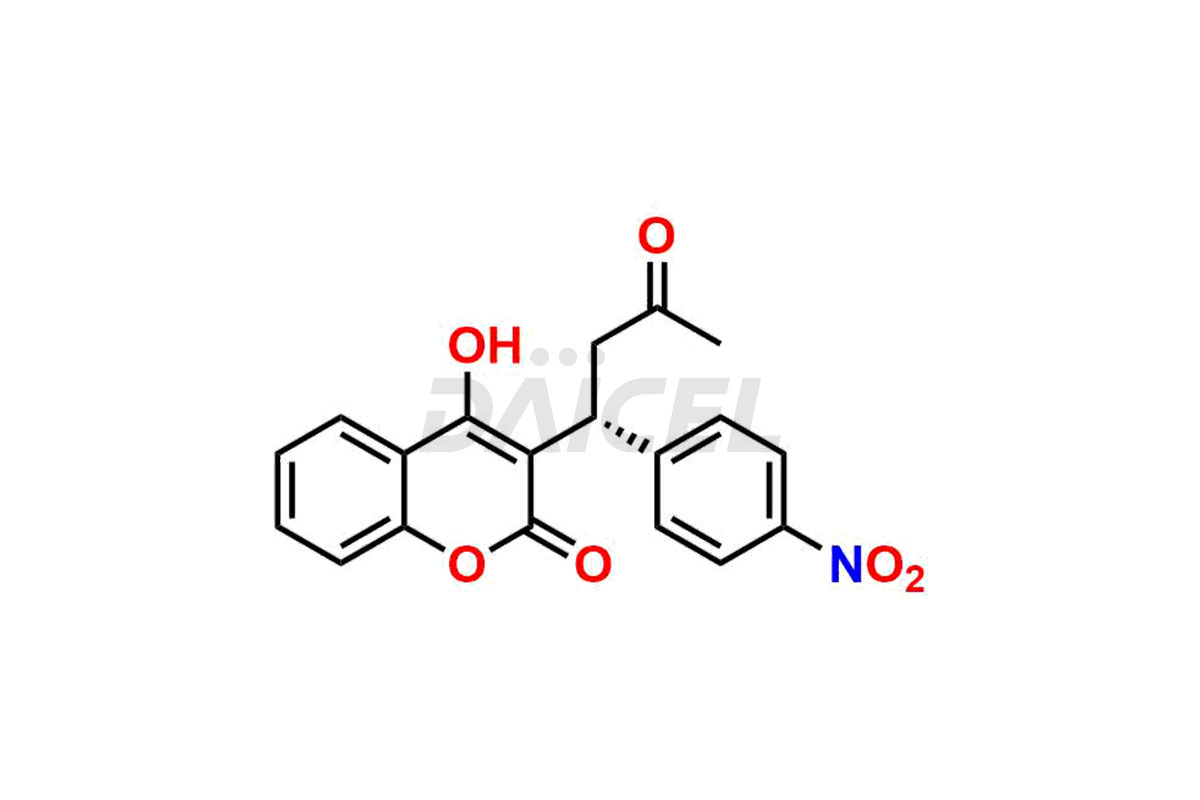
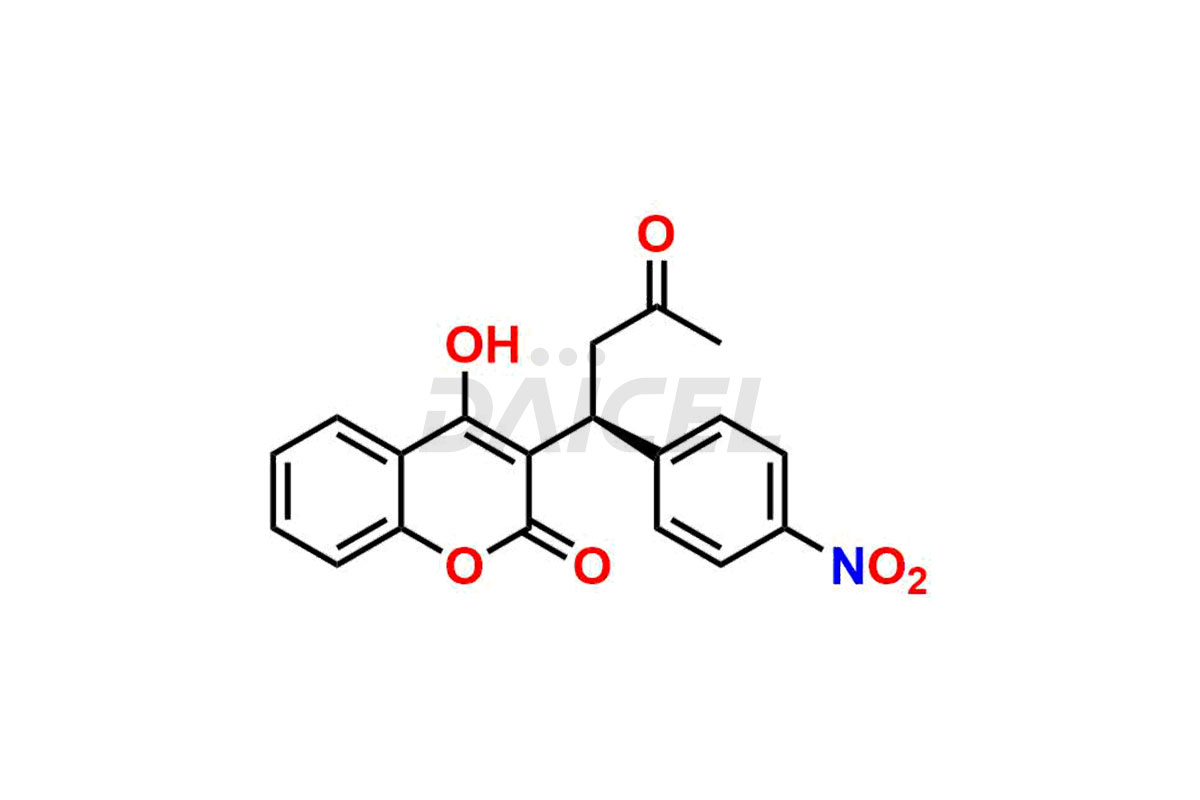
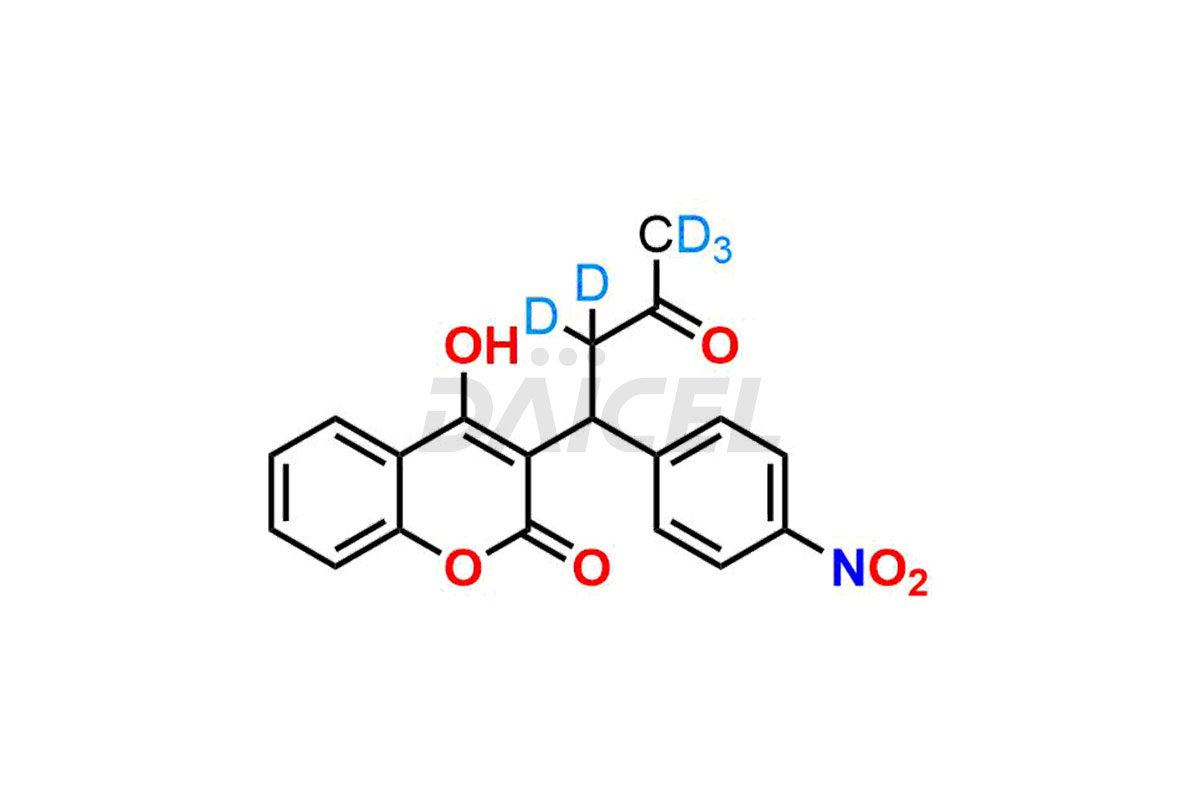
Acenocoumarol
General Information
Acenocoumarol Impurities and Acenocoumarol
Daicel Pharma synthesizes high-quality Acenocoumarol impurities like (R)-Acenocoumarol and (S)-Acenocoumarol, which is crucial in analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient Acenocoumarol. Moreover, Daicel Pharma offers custom synthesis of Acenocoumarol impurities and delivers them globally.
Acenocoumarol [CAS: 152-72-7] is a 4-hydroxycoumarin derivative, an anticoagulant medicine. It antagonizes vitamin K, similar to warfarin, and thus has anticoagulant properties. One advantage of using Acenocoumarol over other anticoagulants, such as warfarin and phenprocoumon, is the shorter duration of action, which is beneficial in case of bleeding.
Acenocoumarol: Use and Commercial Availability
Acenocoumarol is a medicine that treats and prevents thromboembolic diseases, including deep vein thrombosis, pulmonary embolism, cerebral embolism, and thromboembolism in infarction and transient ischemic attacks. In India, the drug is available under the trade names Acitrom and Nistrom.
Acenocoumarol Structure and Mechanism of Action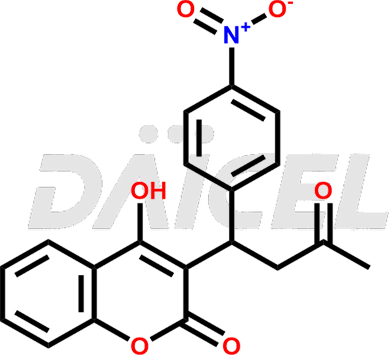
The chemical name of Acenocoumarol is 4-Hydroxy-3-[1-(4-nitrophenyl)-3-oxobutyl]-2H-1-benzopyran-2-one. Its chemical formula is C19H15NO6, and its molecular weight is approximately 353.3 g/mol.
Acenocoumarol inhibits Vitamin K reduction. It prevents carboxylation of glutamic acid residues, Vitamin K-dependent clotting factors.
Acenocoumarol Impurities and Synthesis
During the production of Acenocoumarol, impurities arise from the starting materials, synthetic process, or degradation of the drug substance. These impurities harm the drug’s safety and efficacy, hence monitoring and controlling the level of Acenocoumarol impurities is essential.
Daicel provides a Certificate of Analysis (CoA) for Acenocoumarol impurity standards, (R)-Acenocoumarol and (S)-Acenocoumarol. The CoA is issued from a cGMP-compliant analytical facility and contains complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity1,2. Additional characterization data, such as 13C-DEPT and CHN, can be provided upon request. Daicel can also prepare any unknown Acenocoumarol impurity or degradation product and offer labeled compounds to quantify the efficacy of generic Acenocoumarol. Daicel offers Acenocoumarol-D5, a deuterium-labeled Acenocoumarol standard used in bio-analytical research, such as BA/BE studies. We give a complete characterization report on delivery.
References
FAQ's
References
- Aicha Kraimi, Nasser Belboukhari, Khaled Sekkoum, Abdelkrim Cheriti, Hassan Y Aboul-Enein, Liquid Chromatographic Chiral Separation of Acenocoumarol and Its Hemiketal Form, Journal of Chromatographic Science, Volume 55, Issue 10, November-December 2017, Pages 989–991
- Mandrupkar Supriya, et al, Development of validated stability indicationg RP-HPLC method for estimation of Acenocoumarol in bulk and tablet dosage form, Int. J. Pharm.Sci. Rev. Res/. 16(1), 2012, n 19, 101-106
Frequently Asked Questions
What are the residual solvents in Acenocoumarol?
Residual solvents in Acenocoumarol are volatile substances used during manufacturing and may remain in the final drug product.
How are the impurities in Acenocoumarol detected?
Impurities in Acenocoumarol are detected using analytical techniques such as Reverse-phase high-performance liquid chromatography (RP-HPLC).
Which solvent helps in the analysis of Acenocoumarol impurities?
Acetonitrile is the solvent used in analyzing many impurities in Acenocoumarol.
What are the temperature conditions required to store Acenocoumarol impurities?
Acenocoumarol impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.