LOAD MORE
You're viewed 9 of 14 products
Daicel Pharma synthesizes high-quality Acalabrutinib impurities, ACA-Benzoic acid impurity, ACA-dibutynoyl impurity, ACA-N-oxide impurity 2, Acalabrutinib dimer impurity, Acalabrutinib R-isomer, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Acalabrutinib. Moreover, Daicel Pharma offers custom synthesis of Acalabrutinib impurities and delivers them globally.
Acalabrutinib [CAS:1420477-60-6] is a potential antineoplastic oral medicine. It functions by blocking the activity of Bruton’s tyrosine kinase (BTK), a protein that plays a role in the formation of B cell malignancies.
Acalabrutinib, under the trade name CALQUENCE, is a medicine for treating Mantle Cell Lymphoma (MCL) in adult patients who have already undergone at least one prior therapy. More recently, it is for treating chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma.
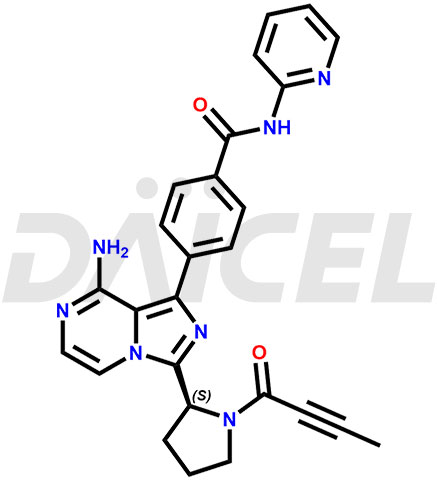
The chemical name of Acalabrutinib is 4-[8-Amino-3-[(2S)-1-(1-oxo-2-butyn-1-yl)-2-pyrrolidinyl]imidazo[1,5-a]pyrazin-1-yl]-N-2-pyridinylbenzamide. Its chemical formula is C26H23N7O2, and its molecular weight is approximately 465.5 g/mol.
Acalabrutinib is a small-molecule BTK inhibitor. It forms a covalent bond with a cysteine residue in the BTK active site, inhibiting BTK enzymatic activity.
During the manufacturing1 of Acalabrutinib, impurities may form as by-products. These impurities are from the starting materials, intermediates, or reaction by-products. The common Acalabrutinib impurities include related substances, degradants, and residual solvents. Some of these impurities may affect the safety and efficacy of the drug product, and thus their levels must be controlled and monitored within acceptable limits.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Acalabrutinib impurities, ACA-Benzoic acid impurity, ACA-dibutynoyl impurity, ACA-N-oxide impurity 2, Acalabrutinib dimer impurity, Acalabrutinib R-isomer, and more. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Acalabrutinib impurity or degradation product. We also provide labeled compounds to quantify the efficacy of Acalabrutinib. Daicel offers Acalabrutinib-D4, a deuterium-labeled Acalabrutinib standard, used in bio-analytical research such as BA/BE studies with isotope data in CoA.
It is vital to control the impurities in Acalabrutinib because they can affect the drug's safety, efficacy, and stability. In addition, regulatory agencies require the identification and control of impurities to ensure the quality of the drug.
The analytical techniques used to detect and quantify the impurities in Acalabrutinib include high-performance liquid chromatography (HPLC), RP-HPLC, liquid chromatography-mass spectrometry (LC-MS), etc.
Acalabrutinib impurities are controlled through various measures, such as optimizing the synthetic process, conducting appropriate analytical testing, and implementing strict quality control measures during manufacturing.
Acalabrutinib impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.