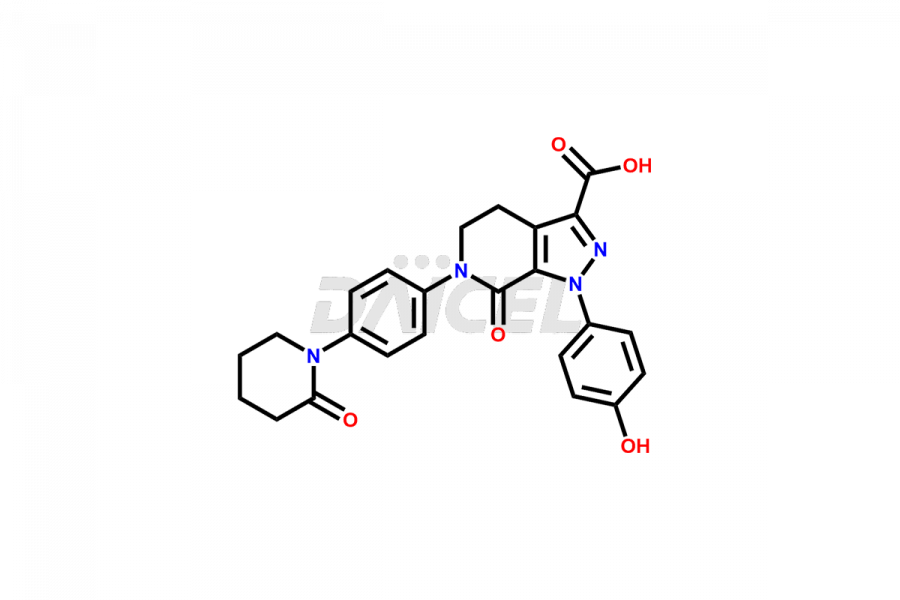
1-(4-hydroxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-y...
- CAT NUMBER DCTI-C-3026
- CAS NUMBER 2459302-74-8
- MOLECULAR FORMULA C24H22N4O5
- MOLECULAR WEIGHT 446.16
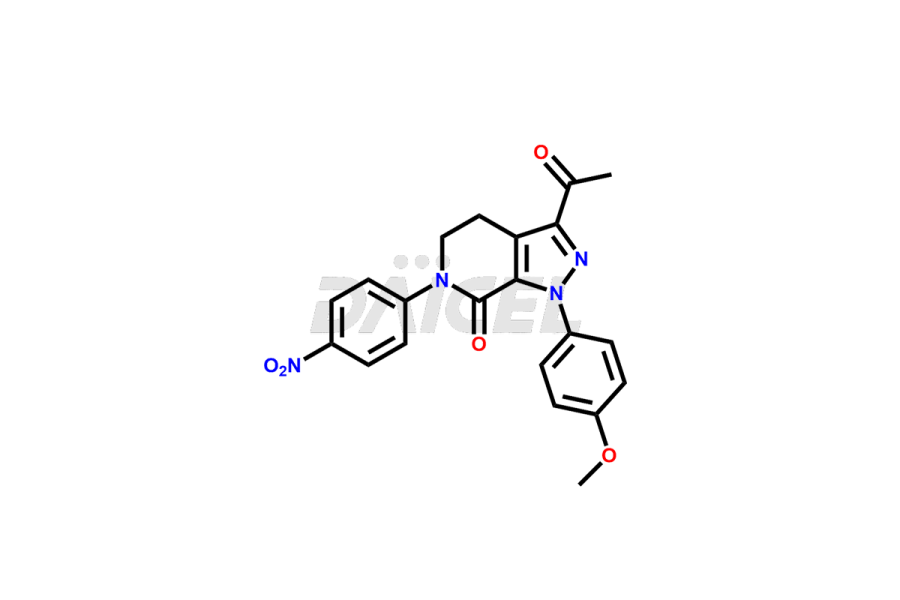
3-acetyl-1-(4-methoxyphenyl)-6-(4-nitrophenyl)-1,4...
- CAT NUMBER DCTI-C-3107
- CAS NUMBER NA
- MOLECULAR FORMULA C21H18N4O5
- MOLECULAR WEIGHT 406.4